Signalment:
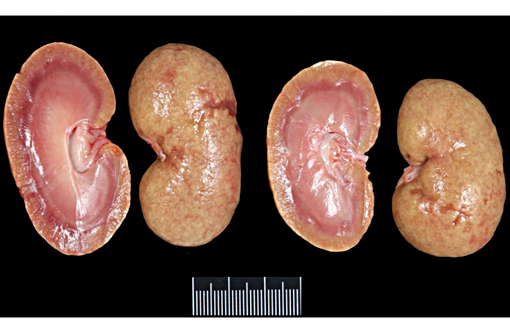
Gross Description:
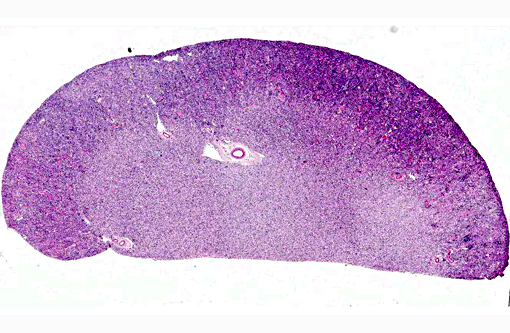
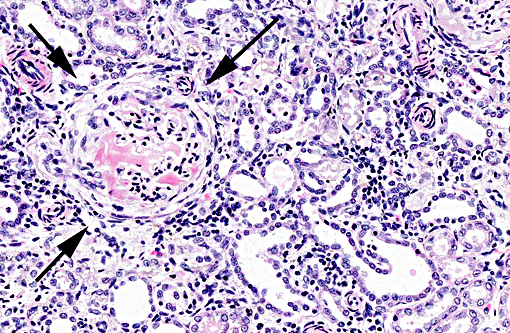
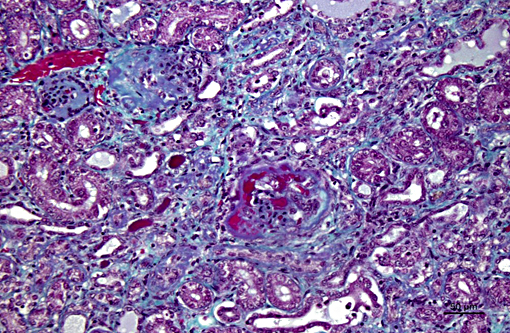
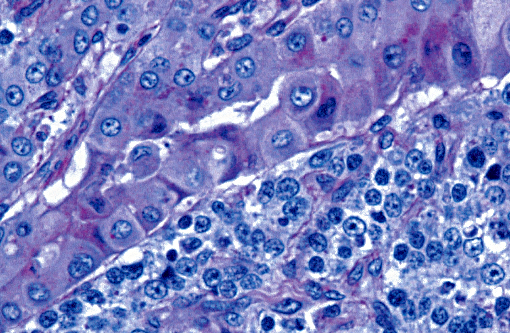
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Hereditary nephropathy (HN) (also called Familial Nephropathy or Hereditary Nephritis) is the most commonly used name for kidney diseases that occur in dogs due to genetic type IV collagen abnormalities.(2,7,10) This group of diseases is considered analogous to human Alport nephropathy, although the latter presents usually with ocular and/or hearing abnormalities which have not been reported in dogs .(9,3-6) To date, 4 different collagen IV gene mutations have been identified in dogs with HN (table 1). HN in the English Cocker Spaniel is an invariably progressive and ultimately fatal renal disease, which typically causes renal failure between 6 months and 2 years of age. The disease is inherited as an autosomal recessive trait and based on a genetic defect caused by a single base substitution in the exon 3 of COL4A4 gene (A → T).(2) Collagen (type IV) defects cause severe structural and functional alterations in glomerular basement membranes (GBM).(7,4,5) Unlike most colla-gens, type IV collagen occurs only in the basement membranes (BM) and comprises up to six genetically distinct α-chains (designated as α1(IV) to α6(IV)) encoded by 6 genes (COL4A1 to COL4A6).(6) Three defined trimer combinations (α1α1α2, α3α4α5 and α5α5α6) are formed from the six α(IV) chains.(5-7) The α1 and α2 chains are present in the BM of all tissues, whereas the other four chains have restricted tissue distribution during the development.(5,6) The expression of collagen IV chains is also subjected to temporal regulation.(6) In the GBM, genes encoding the α1 and α2 chains are expressed during embryonic development, but their levels gradually decrease as the expression of genes encoding the α3, α4, and α5 chains starts. This switch explains why the affected animals are born apparently healthy and have an early onset of disease. In affected English Cocker Spaniel dogs, the mutation in COL4A4 prevents a normal synthesis of α4 chains and therefore inhibits a normal assembly of α3α4α5 collagen type IV heterotrimers in mature glomeruli. Instead, these heterotrimeric units are replaced by others lacking α4, which are suggested to be structurally weaker or less resistant to proteolytic degradation. These structural changes in the GBM lead to glomerular damage due to abnormal cell-cell and matrix-cell interactions, and a progressive tubule-interstitial injury.(7)
Affected English Cocker Spaniel puppies typically present with proteinuria as their first clinical manifestation of the disease at the age of 2 to 8 months. Renal damage progresses and end-stage renal disease is often present at 12 months of age (ranging from 6 to 18 months), as was the case in the dog here presented.(7)
| Breed | Mode of inheritance | Gene affected | Location within gene | Specific mutation | Reference |
| Samoyed | X-Linked | COL4A5 | Exon 35 | Single nucleotide substitution (G → T) | 9 |
| Mixed breed from Navasota, Texas (USA) | X-Linked | COL4A5 | Exon 9 | 10-base pairs deletion | 10 |
| English Cocker Spaniel | Autosomal recessive | COL4A4 | Exon 3 | Single nucleotide substitution (A → T) | 1 |
| English Springer Spaniel | Autosomal recessive | COL4A4 | Exon 30 | Single nucleotide substitution (C → T) | 11 |
Contributors were unable to investigate a possible familiar occurrence of renal disease in the kindred of the dog presented here, since no information about it could be obtained.
The light microscopic lesions in the kidney of HN affected dogs are reported to be segmental thickening of GBMs and mesangial expansion, progressing through glomerular and periglomerular fibrosis and glomerulosclerosis. Usually, these lesions are accompanied by tubulointerstitial nephritis, interstitial fibrosis and tubular atrophy.(7,10) All these light histological features were observed in the present case. The striking presence of extracellular material observed within the Bowmans spaces of most glomeruli is unusual in the contributors opinion. Based on its appearance in hematoxylin and eosin (HE) and the performed special stains, the contributors interpret this material as a very protein rich glomerular filtrate.
Immunohistochemistry (IHC) and transmission electron microscopy (TEM) are useful techniques to further characterize the lesion, but they were not performed in the present case. Normal mature GBM is known to strongly express α3, α4 and α5 chains of type IV collagen. In English Cocker Spaniels with HN, however, the expression of those chains is either absent (α4) or greatly reduced (α5). Additionally, there is increased expression of three other chains (α1, α2, and α6) that would normally only be minor components of the mature GBM and therefore weakly expressed.(7) Ultrastructural GBM changes are common to all the genetically characterized examples of HN. The principal change is an irregular thinning or thickening of GBM with splitting and fragmentation of its lamina densa. There is also often fusion of visceral epithelial cell foot processes and wrinkling of glomerular capillary walls.(7,8) In this case, the definite diagnosis was based on a DNA test offered by ANTAGENE in France. Genetic tests are recommended in cases of suspected HN in English Cocker Spaniels and are widely available (OptiGen, Laboklin, Genetic Technologies, ANTAGENE, Genindexe, Genomia, Van Haeringen)
(www.thekennelclub.org.uk/media/14688/dnatestsworldwide.pdf#sthash.eQZ2iMgB.dpuf).
JPC Diagnosis:
Conference Comment:
Nonspecific changes which occur in the tubules and interstitium in glomerular disease include tubular degeneration, atrophy, interstitial fibrosis and nephritis, as was described in this case. These secondary changes tend to increase in severity as the primary glomerular disease progresses. Although the exact mechanism resulting in the tubular and interstitial changes in this condition is not completely understood, the underlying process is presumed to be similar for the development of tubular lesions in other glomerular disorders.(7) Blood is supplied to the tubules via the vasa recta from the efferent glomerular arteriole; in cases of glomerulosclerosis, this blood flow through the vasa recta is decreased, resulting in hypoxia and subsequent tubular epithelial cell death. Damaged tubules thereupon become lined by cuboidal or squamous cells which do not have a brush border and lack normal tubule cell function. Chronic proteinuria is also reported to damage tubular epithelium.(11)
In the initial stages of HN, biopsies from affected dogs presenting with proteinuria can be normal. Early histologic changes include mild focal and segmental mesangial expansion, and the lesions eventually progress to global glomerulosclerosis as described above. The distinctive features of this entity are demonstrated via electron microscopy, as well as with IHC that is specific for the different types of type IV collagen alpha chains. Tissue IHC is necessary to demonstrate the presence of the abnormal collagen chains because not all dogs that have ultrastructural changes consistent with HN have evidence of abnormal type IV collagen. Although proteinuria is a classic clinical manifestation of this condition and moderate hypoalbu-minemia is seen, affected animals usually do not have nephrotic syndrome or hypertension, but rather develop a worsening uremia as the condition progresses.(7)
References:
1. Cox ML, Lees GE, Clifford EK, Murphy KE. Genetic cause of X-linked Alport syndrome in a family of domestic dogs. Mamm Genome. 2003;14(6):396-403.
2. Davidson AG, Bell RJ, Lees GE, Kashtan CE, Davidson GS, Murphy KE. Genetic cause of autosomal recessive hereditary nephropathy in the English Cocker Spaniel. J Vet Intern Med.
2007;21(3):394-401.
3. Deltas C, Pierides A, Voskarides K. Molecular genetics of familial hematuric diseases. Nephrology Dialysis Transplantation. 2013;28(12):2946-60.
4. Gubler MC. Inherited diseases of the glomerular basement membrane. Nat Clin Pract Nephrol. 2008;4(1):24-37.
5. Heidet L, Cai Y, Guicharnaud L, Antignac C, Gubler M-C. Glomerular Expression of Type IV Collagen Chains in Normal and X-Linked Alport Syndrome Kidneys. Am J Pathol. 2000;156(6):1901-1910.
6. Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71(5):357-70.
7. Lees GE. Kidney diseases caused by glomerular basement membrane type IV collagen defects in dogs. J Vet Emerg Crit Care. 2013;23(2):184-93.
8. Lees GE, Wilson PD, Helman RG, Homco LD, Frey MS. Glomerular ultrastructural findings similar to hereditary nephritis in 4 English cocker spaniels. J Vet Intern Med. 1997;11(2):80-5.
9. Longo I, Porcedda P, Mari F et. al. COL4A3/COL4A4 mutations: from familial hematuria to autosomal-dominant or recessive Alport syndrome. Kidney Int. 2002;61(6):1947-56.
10. Maxie GM and Newman SJ. Urinary system. In: Pathology of Domestic Animals. Jubb, Kennedy, and Palmer's. 5th edition, Saunders Elsevier. 2007; Vol.2, p. 460.
11. Newman SJ. Urinary System. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Mosby Elsevier; 2012:590-627.
12. Nowend KL, Starr-Moss AN, Lees GE, Berridge BR, Clubb FJ, Kashtan CE, Nabity MB, Murphy KE. Characterization of the Genetic Basis for Autosomal Recessive Hereditary Nephropathy in the English Springer Spaniel. J Vet Intern Med. 2012;26(2):294-301.
13. Zheng K, Thorner PS, Marrano P, Baumal R, McInnes RR. Canine X chromosome-linked hereditary nephritis: a genetic model for human X-linked hereditary nephritis resulting from a single base mutation in the gene encoding the alpha 5 chain of collagen type IV. Proc Natl Acad Sci U S A. 1994;91(9):3989-3993.