Signalment:
Gross Description:
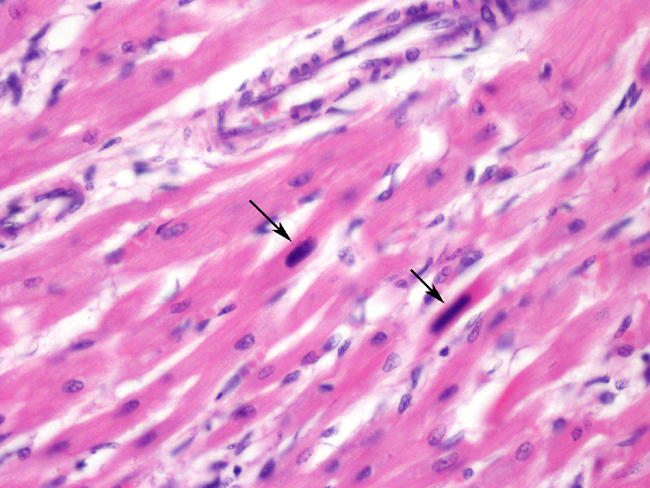
Histopathologic Description:
There is moderate to severe, multifocal to coalescing, interstitial, predominantly lymphocytic and histiocytic infiltration with occasional scattered plasma cells and neutrophils, together with mild to moderate interstitial fibrosis. The myocardial fibers exhibit moderate diffuse anisocytosis and multifocal loss of striation, with cytoplasmic eosinophilia and occasional fragmentation. Multifocally, single, large (up to approximately 20x40 _m), homogeneous and occasionally stippled, basophilic, mostly elongated, intranuclear inclusion bodies <1-1) are observed in myofibers. In the cells containing inclusion bodies, the nuclear chromatin is clumped at the nuclear membrane.
Morphologic Diagnosis:
Condition:
Contributor Comment:
Parvoviridae are amongst the smallest DNA viruses, the virion being 18 to 26 nm in diameter, and are composed entirely of protein and DNA.11 The lack of fatty components, typically present in viral envelopes, makes these viruses stable under diverse environment conditions and difficult to eliminate through disinfection. They can however, be inactivated by bleach, formalin and sunlight. 7
The host range is one of the widest, the subfamily Parvovirinae infecting vertebrates, including humans and the subfamily Densovirinae infecting insects. CPVs infect domestic dogs and several species of wild canids, including coyotes, bush dogs, gray wolves, racoon dogs and manned wolves. Presently two autonomous parvoviruses are known to infect dogs: CPV type 1 and CPV type 2.Â
CPV type 1 was initially named Minute Virus of Canines and was first identified in 1970.1 It was proven to cause enteritis and diarrhoea in neonatal canines. It appears to be highly related to the Bovine Parvovirus 14 and is antigenically unrelated to CPV type 2. To date, only few cases have been reported.
On the other hand, CPV type 2 appeared as a pandemic disease and presented characteristics of an epidemic infection affecting dogs of all ages. Although there is no specific data to support this, we can assume from the low mortality rate, that death was mostly restricted to canines less than 4 months old. 3 It was first detected in 1978 but is estimated to have emerged up to 10 years before. 10
CPV type 2 is thought to result from mutations of feline panleukopenia parvovirus (FPV), with which it shares more than 98% of the DNA sequence 4 or from a closely related carnivore parvovirus.12 CPV type 2 was found to be unable to infect cats although it was able to replicate in vitro in feline cells.13 As opposed to this, FPV was found able to replicate in vivo in the canine thymus but unable to replicate in vitro in canine cultured cells.
The CPV type 2 strain was soon replaced, worldwide, by two new lineages: CPV2a, identified in 1980, that later gave rise to CPV2b, identified in 1984, both with the added ability to replicate in cats and produce clinical signs in experimentally infected cats.13,18 In Italy, Vietnam and Spain, a third type, CPV2c has been identified. 10 This antigenic drift is accompanied by successive replacement of prevalent strains by newer serotypes.13 This is exemplified by the gradual disappearance of CPV2b from the dog population in Italy 6, 10 and the replacement of CPV2a by CPV2b in the UK.5
The mutations occur at the level of the VP1/VP2 gene (encoding capsid proteins) and the new serotypes have an improved binding ability to its receptor, the canine transferrin receptor.4
CPVs require host cells to be in the S-phase to replicate as they are unable to induce it. They replicate within the nuclei of infected cells and are highly dependent on the cellular function.17
Pathology and clinical signs are dependent on the time of infection and which cells are in a highly mitotic rate at that particular moment within the host. Infection occurs oronasally and disease develops after 3 to 10 days of incubation.6 After faeco-oral infection, the virus is taken up by the epithelium over the tonsils and Peyers patches. In 1 to 2 days after experimental inoculation, the virus can be found in the mesenteric lymph nodes. Further dissemination of virus particles into other central or peripheral lymphoid tissues occurs via infected lymphoblasts. Following the lysis of infected cell, viruses are released and contribute to elevate the viremia which is only terminated if neutralising antibodies appear, typically 5 to 7 days post infection. Moderate pyrexia usually occurs.2
If the infection takes place up to two weeks postnatally and the puppies do not have sufficient neutralising antibodies, nonsuppurative myocarditis is the most common condition. On the other hand, if infection occurs later than these two weeks, due to the fast replication of the epithelia of the small intestines and bone marrow granulopoiesis, hemorrhagic gastroenteritis with lymphoid depletion is the pathological picture. The two forms of the disease rarely occur at the same time in an individual or group of animals. When nonsuppurative myocarditis occurs, it is usually detectable between the third and eighth week of life, but can be asymptomatic until animals are six months old. Puppies frequently succumb to sudden death but can also present symptoms of congestive heart failure due to myocardial scarring or conduction failure. Grossly, the main findings are cardiomegaly and lesions in the myocardium, more pronounced in the left atrium and left ventricle. Pericardial and pleural effusions, ascites and hepatomegaly can also be observed. In the myocardium lesions are pale and streaky and often accompanied by multifocal petechial.8 Microscopically, single, homogeneous, basophilic or amphophilic, roundish to elongated, intranuclear, Feulgen-positive inclusion bodies are observed.2, 8 The chromatin is clustered at the nuclear membrane. Inclusion bodies are more frequent in late incubation, before extensive exfoliation (in the intestinal form) or infected cell lysis. In animals 4 to 7 weeks old, separation of the thin myocytes by extracellular oedema, histiocytes, fibroblasts and fibrous tissue is seen. Myocytes appear granulated and with fragmented cytoplasm. In animals 6 to 9 weeks old, inflammation is more severe and mainly lymphoplasmacytic. In juvenile animals, 14 to 24 months old, inflammation is milder, histiocytes and fibroblasts are more frequent, and fibrosis more extensive.
JPC Diagnosis:
Conference Comment:
Intranuclear inclusions are usually observed late in the incubation phase and prior to the lysis or exfoliation of the cells. Therefore it is possible that intranuclear inclusions may not be seen in samples submitted for histopathology.2
Other potential causes of myocarditis in canines include:8,19
Viral: Morbillivirus (canine distemper)
Parasitic: Neospora caninum, Trypanasoma cruzi, Toxoplasma gondii
Ricketsial: Rickettsia rickettsii, Ehrlichia canis, Bartonella elizabethae
Fungal/Algae like: Prototheca sp.
Spirocheatal: Borrelia burgdorferi
Other parvoviruses in animals include porcine parvovirus (SMEDI); feline parvovirus (feline panleukopenia); rat parvovirus (Kilham rat virus); minute virus of mice; goose parvovirus; and two genetically and antigenically distinct parvoviruses in mink (mink enteritis virus, which causes similar lesions as feline parvovirus, and Aleutian mink disease virus, which causes immune complex glomerulonephritis and arteritis).16
References:
2. Brown CC, Baker DC, Barker IK: Alimentary system. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 4th ed., vol. 2, pp. 177-178. Elsevier Limited, St. Louis, MO, 2007
3. Carmichael L: An annotated historical account of canine parvovirus. J Vet Med B Infect Dis Vet Public Health 52:303-311, 2005
4. Chang S, Sgro J, Parrish C: Multiple aminoacids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J Virol 66:6858-6867, 1992
5. Davies M: Canine Parvovirus strains in the UK. Vet Rec 160:416, 2007
6. Decaro N, Martella V, Elia G, Desario C, Campolo M, Lorusso E, Colaianni ML, Lorussi A, Buonavoglia C: Tissue distribution of the antigenic variants of canine parvovirus type 2 in dogs. Vet Microbiol 121:39-44, 2007
7. Ettinger SJ, Feldman EC: Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat, 6th ed., pp. 646-647. Elsevier Saunders, St. Louis, MO, 2005
8. Fox PR, Sisson D, Mo_se NS: Textbook of Canine and Feline Cardiology: Principles and Clinical Practice, 2nd ed., pp. 832-833. Saunders, Philadelphia, PA, 1999
9. Gelberg, HB: Alimentary system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 378-379. Elsevier, St. Louis, MO, 2007
10. Martella V, Cavalli A, Pratelli A, Bozzo G, Camero M, Buonavogluia D, Narcisi D, Tempesta M, Buonavoglia C: 2A Canine Parvovirus mutant is spreading in Italy. J Clin Microbiol 42:1333-1336, 2004
11. Muzyczka N, Berns KI: Parvoviridae: the viruses and their replication. In: Fields Virology, eds. Knipe DM, Howley PM, 4th ed., vol 2, pp. 2327-2352, Lippincott Williams & Wilkins, Philadelphia, PA, 2001
12. Parrish C, Evermann J, Carmichael L: Natural variation of canine parvovirus. Science 230:1046-1048, 1985
13. Parrish CR, Aquadro CF, Strassheim ML, Evermann JF, Sgro J-Y, Mohammed HO: Rapid antigenic replacement and DNA sequence evolution of canine parvovirus. J Virol 65:6544-6552,1991
14. Schwartz D, Green B, Carmichael LE, Parrish CR: The canine minute virus (minute virus of canines) is a distinct parvovirus that is most similar to bovine parvovirus. Virology 302:219-223, 2002
15. Shackelton L, Parrish C, Truyen U, Holmes E: High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc Natl Acad Sci USA 102:379-84, 2005
16. Steinel A, Parrish C R, Bloom M E, and Truyen U: Parvovirus infections in wild Carnivores. J Wildl Dis 37:594-607, 2001
17. Truyen U: Evolution of canine parvovirus--a need for new vaccines? Vet Microbiol 117:9-13, 2006
18. Truyen U, Evermann JF, Vieller E, Parrish C: Evolution of canine parvovirus involved loss and gain of feline host range. Virology 15:186-189, 1996
19. Van Vleet JF, Ferrans VJ: Cardiovascular system. In: Pathologic Basis of Veterinary Disease, eds. McGavin MD, Zachary JF, 4th ed., pp. 591-593. Elsevier, St. Louis, MO, 2007