CASE IV: 21-199 (JPC 4167919)
Signalment:
Male, Warmblood, fetus, ~ 180-210 days gestation (estimated)
History:
Fetus found in the morning with mare having aborted overnight. No preceding clinical signs. Mare had Potomac horse fever in the fall. No other recent changes, closed herd.
Gross Pathology:
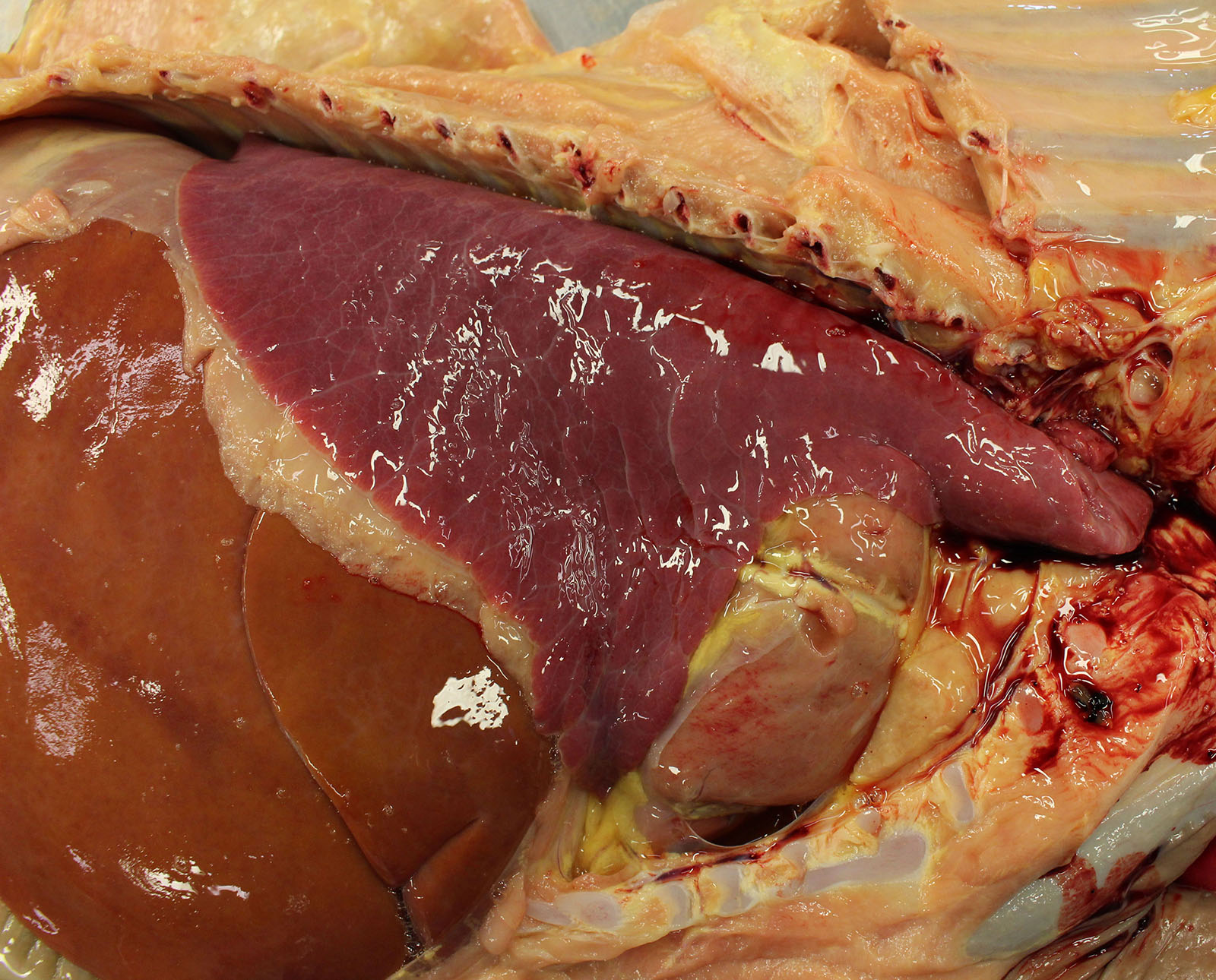
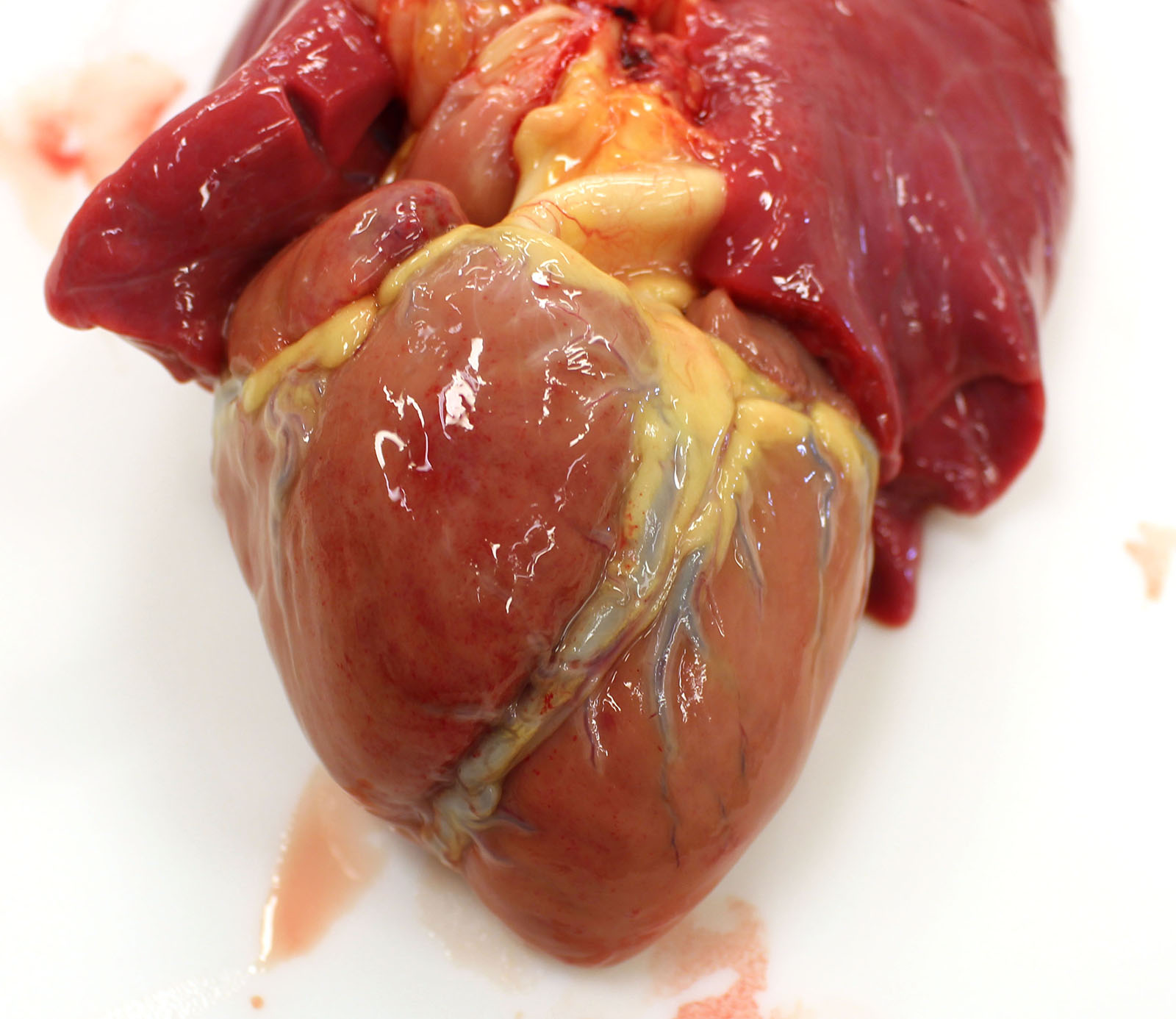
Gross examination of the submitted fetus (estimated gestational age of 180 to 210 days) and associated placenta revealed evidence of enlarged/non-collapsed, mildly firm, lungs and faint, generalized, myocardial pallor. The liver is enlarged and pale and dotted with faint, pin-point foci of pallor. Lymphoid follicular hyperplasia was within the splenic white pulp accompanied by generalized lymphadenomegaly and an enlarged pale thymus.
Laboratory Results:
EHV 1 and 4 PCR (pooled lung, liver, and spleen) ? Negative
Potomac Horse Fever PCR (pooled lung, liver, and placenta) - Positive
Microscopic Description:
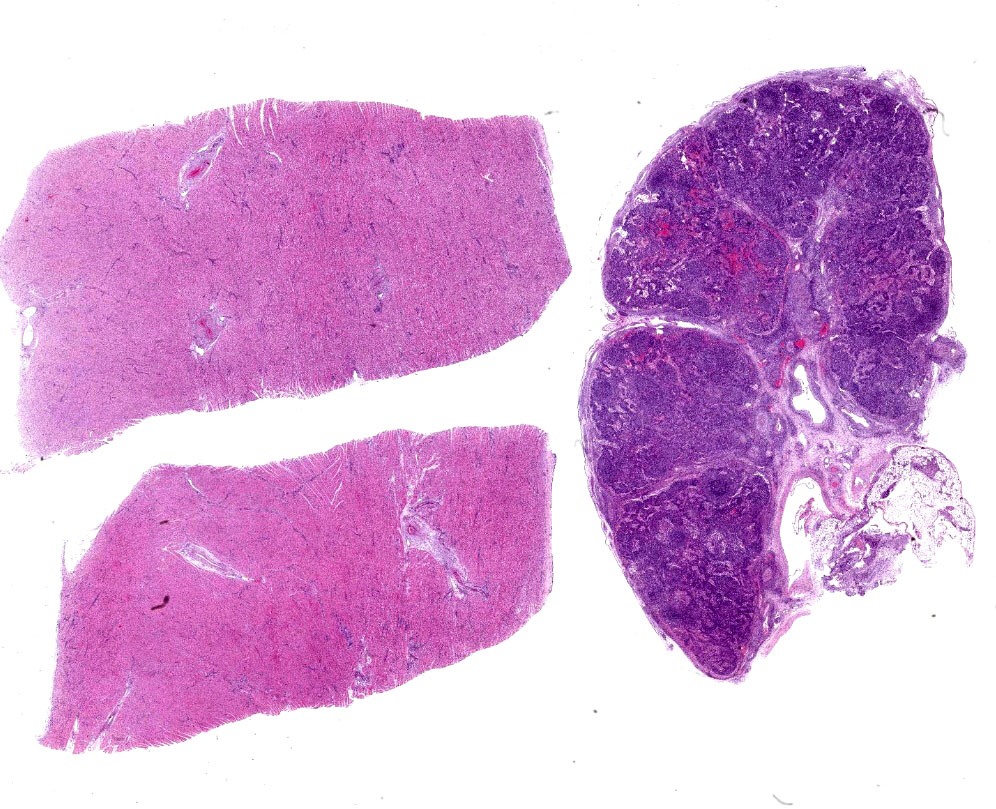
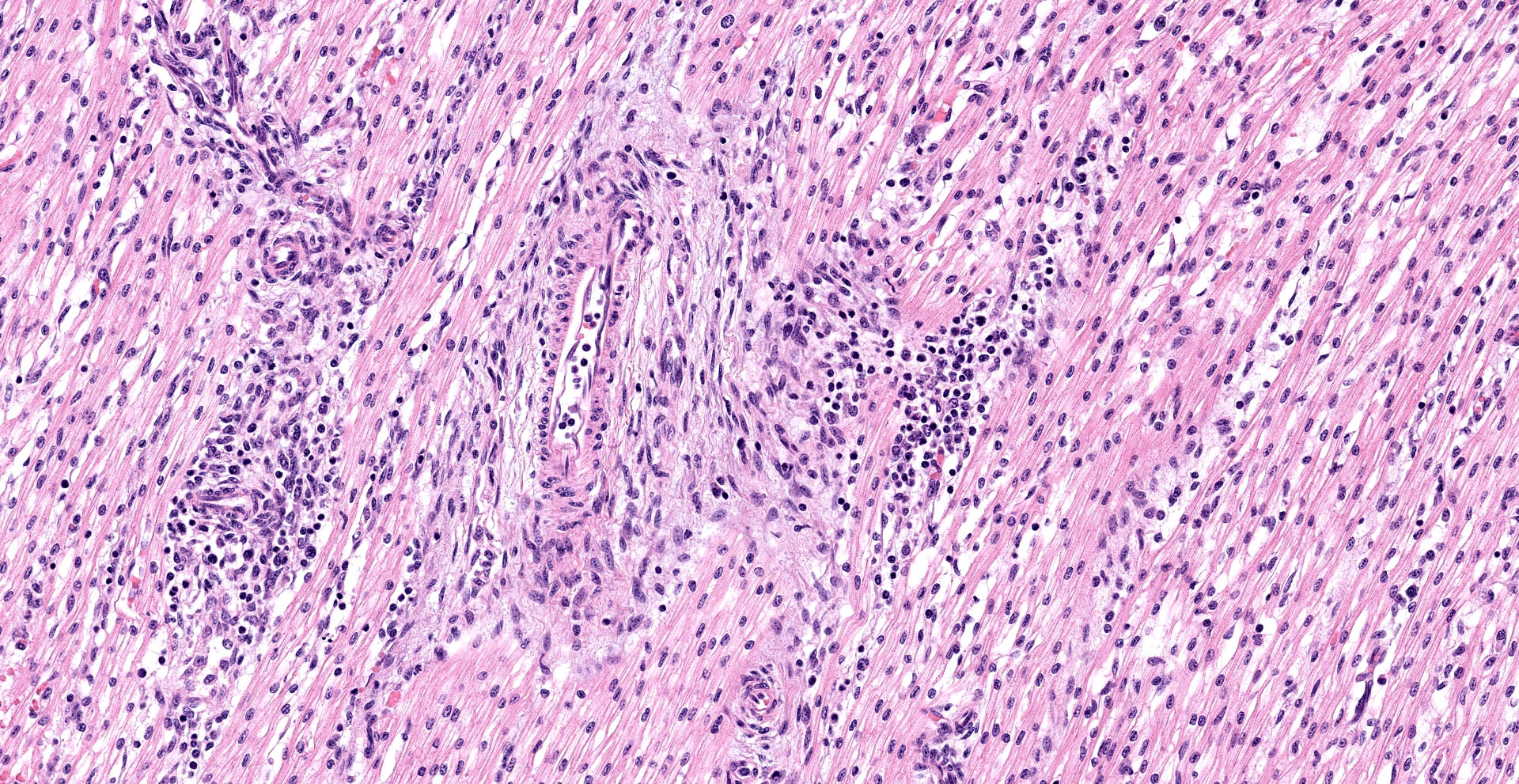
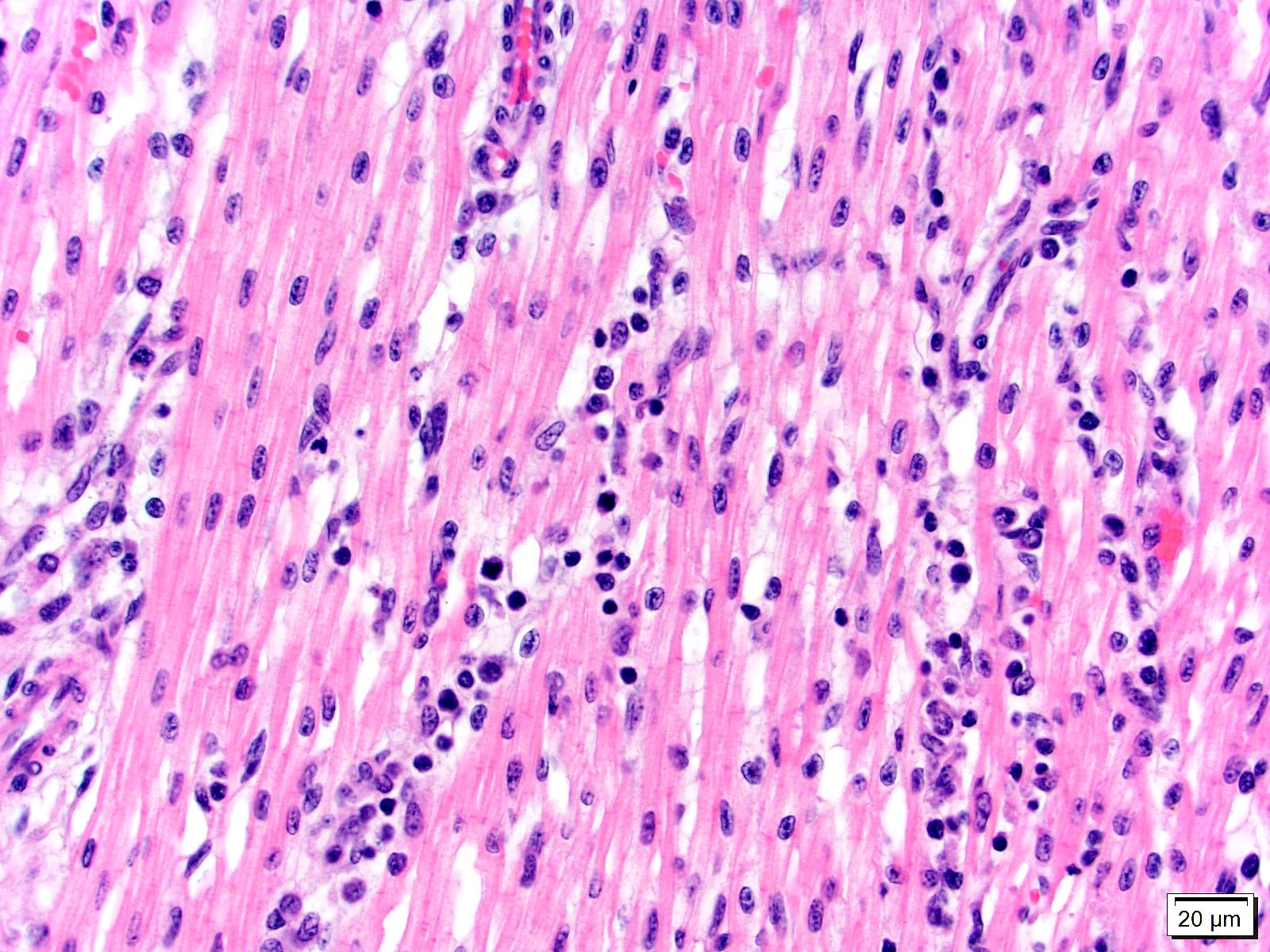
Heart, left and right ventricles: Sections of left and right ventricular myocardium are examined, and both contain similar changes. The sections are dotted with moderately cellular and often vasocentric foci of inflammation. The perivascular to interstitial infiltrates consist of lymphocytes, macrophages, fewer plasma cells and rare neutrophils. The inflammatory cells often infiltrate the vessel walls (interpreted as lymphohistiocytic vasculitis) and are accompanied by mild to moderate expansion of the vascular adventitia, and adjacent myocardial interstitium, by clear edema fluid.
The endothelial cells lining affected vessels, and interstitial fibroblasts, are plump with vesicular nuclei (interpreted as reactive change). The cardiomyocytes separated by inflammatory cells and attendant edema are frequently pale with perinuclear clearing to vacuolation and plump/vesicular nuclei (degenerative change suggestive of cardiomyocyte injury). Similar inflammatory infiltrates are noted within the lining epicardium.
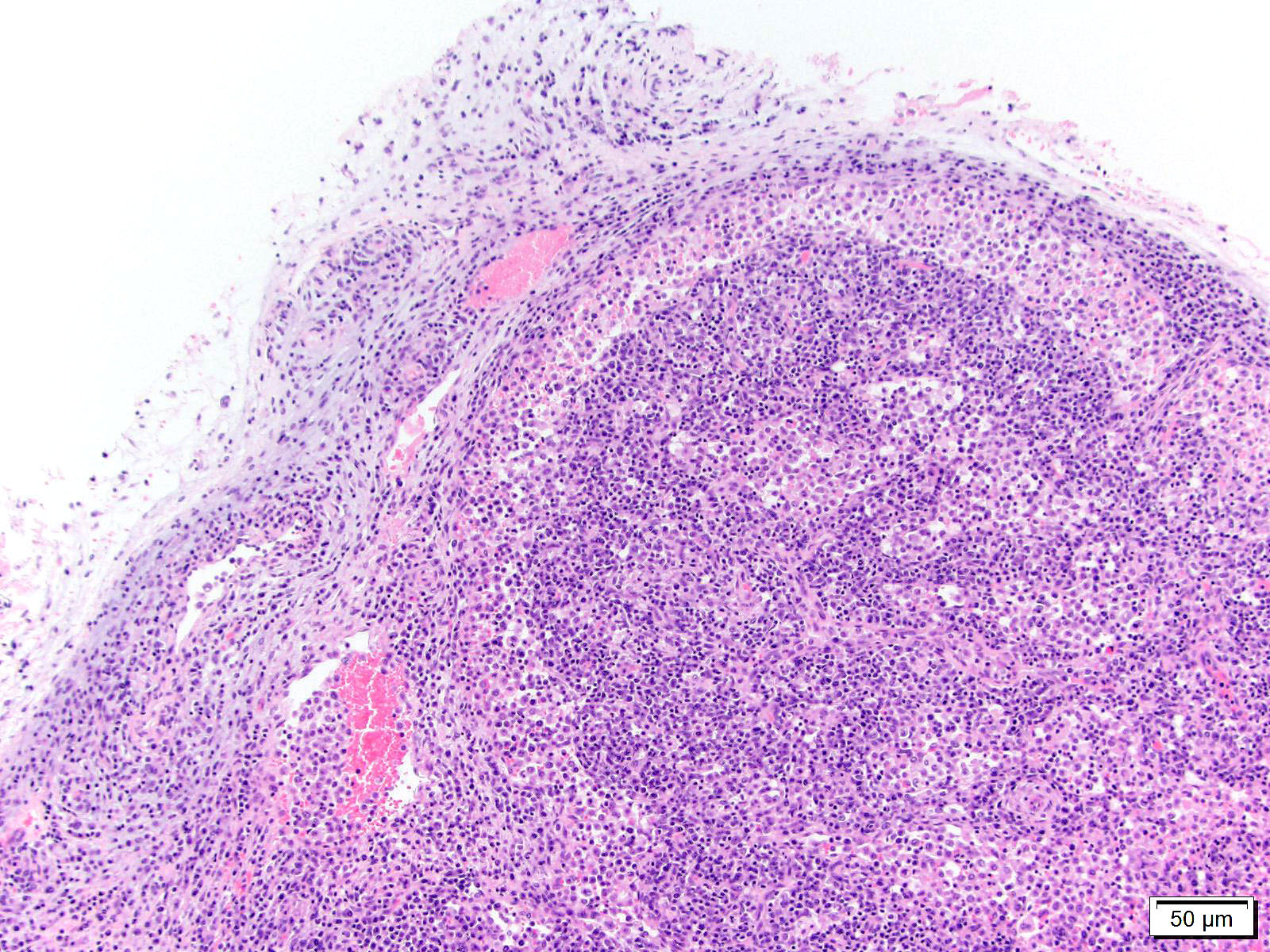
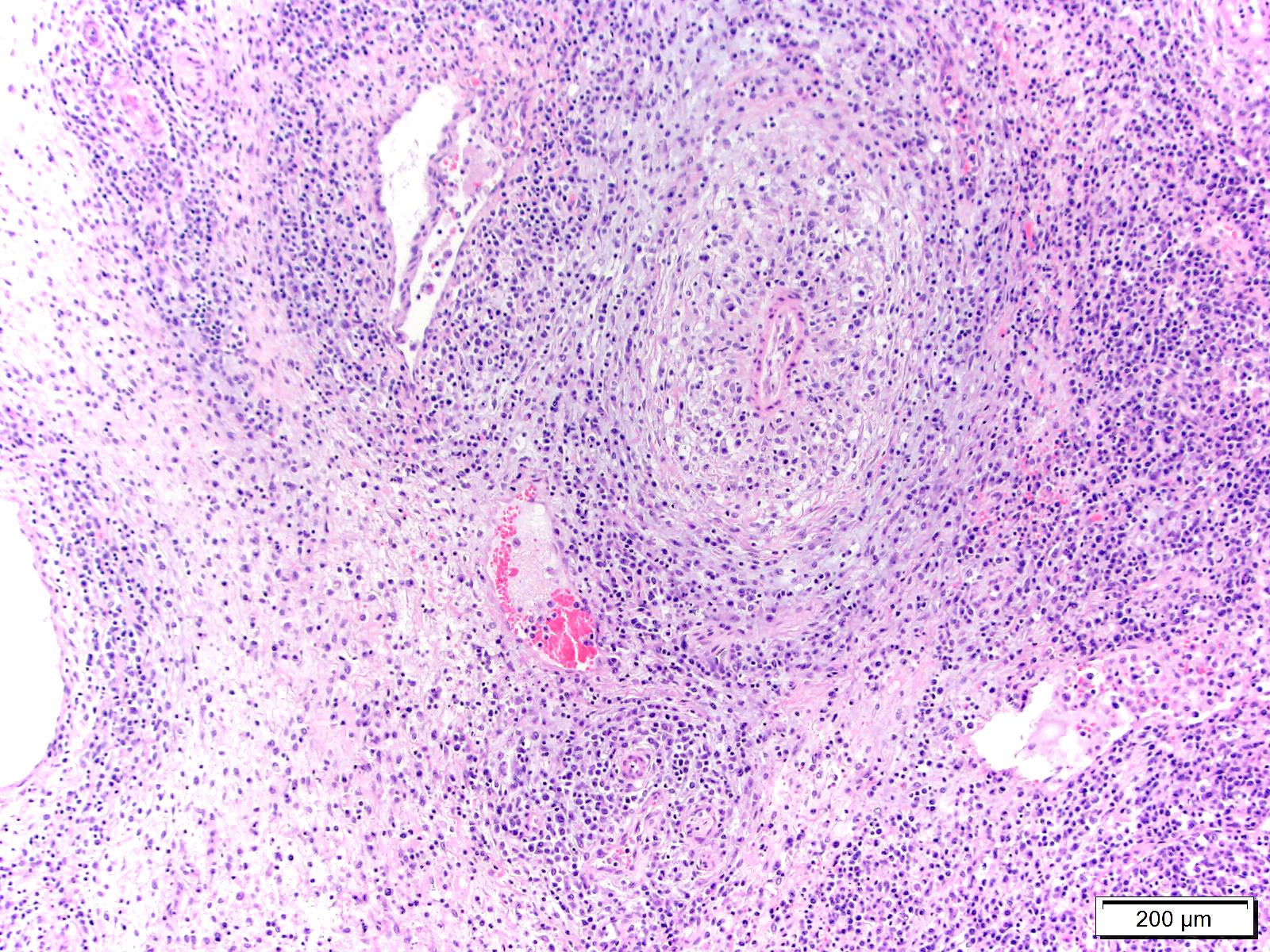
Lymph node, mediastinal: The capsule as well as capsular and hilar vessels are expanded and infiltrated by variable dense inflammatory infiltrates composed predominantly of lymphocytes and macrophages. Occasional vessels contain luminal aggregates of eosinophilic fibrillar material (interpreted as fibrin) accompanied by marginated and subintimal inflammatory cells. The cortices are dotted with prominent secondary follicles (follicular hyperplasia) similar edema and variably dense lymphohistiocytic infitrates noted within the supporting capsule, interstitium, and vascular adventitia. The subcapsular and medullary sinuses are moderately distended with mixtures of acute hemorrhage, circulating mononuclear cells, and foamy to debris-laden macrophages. Subjectively, increased numbers of plasma cells distend the medullary cords. Similar vasocentric foci of inflammation are noted amongst the surrounding adipose tissue. Some sections of lymph node also contained extensive regions of acute necrosis.
Additional histopathological findings:
Small intestine: Lymphohistiocytic enteritis, moderate, subacute
Lung: Interstitial pneumonia, lymphohistio-cytic, moderate, subacute, diffuse
Liver: Lymphohistiocytic portal hepatitis, mild, diffuse
Kidney: Lymphohistiocytic interstitial nephritis, moderate, diffuse with acute tubular injury and necrosis
Adrenal gland: Lymphohistiocytic adrenal-itis, perivascular and interstitial, moderate, subacute
Spleen, white pulp: Follicular hyperplasia, moderate
Contributor's Morphologic Diagnoses:
1. Heart, left and right ventricle: Interstitial myocarditis and vasculitis, lymphohistiocytic, multifocal, subacute, moderate with perivascular and interstitial edema.
2. Lymph node, mediastinal: Vasculitis and lymphadenitis, lymphohistiocytic, multifocal, subacute, with cortical follicular hyperplasia.
Contributor's Comment:
The lesions found within the heart, liver, lymph node, and intestine of this aborted fetus are characteristic pathological findings described in fetuses aborted due to an underlying Neorickettsia risticii infection (Potomac horse fever). Typical necropsy findings found non-pregnant horses dying from Potomac horse fever (PHF) include enterocolitis with abortion occurring in pregnant mares following clinical recovery. Abortions due to PHF have been reported to occur anywhere from between 166- to 226-days gestation, with clinical illness being reported anywhere from 68- to 143-days of gestation. Clinical signs reported in the pregnant mares, prior to abortion, are similar to those reported in nonpregnant horses (gastrointestinal hypermotility or diarrhea, fever, dehydration, depression, tachypnea, tachycardia, and anorexia). Neorickettsia risticii is not included in the listed causes of equine abortion in several large case reviews5,6,8 and textbooks15 related to equine abortion and neonatal loss, despite the few reports being found in the literature.3,9
The Neorickettsia spp. bacteria replicate intracellularly within host cells (within a phagolysosome) and often use trematode hosts. Various trematode species (affecting all levels of the life cycle) found throughout Canada and the United States act as hosts for this bacterium with the trematodes often being situated within their own insect (e.g. damselflies, caddisflies, stoneflies, mayflies, etc.) or snail hosts.11,12,13 Infection is thought to occur most commonly in horses housed around creeks or other bodies of water through accidental ingestion of snails or aquatic insects harboring N. risticii-infected trematodes. Following ingestion, the incubation period is approximately 9 to 14 days and diarrhea typically 1 to 3 days following the onset of fever. Many cases are subclinical with some horse developing clinical disease of PHF. It is thought that this and similar rickettsial organisms has an affinity for monocytes and intestinal epithelial cells4 with infection sometimes resulting in cellular injury/necrosis and attendant lymphohistiocytic inflammation. The mechanism through which the bacteria inoculate the target cells (monocytes and enterocytes) is not known but may resemble that of Neorickettsia helmonthecia in cases of salmon poisoning disease in dogs. Infection via inoculation of enterocytes and circulation monocytes may account for the bacteria dissemination with enteric and vasocentric lesions found in the intestine and a variety of organ tissues, including the heart, liver, lung, spleen, lymph nodes, and adrenal gland in fetal tissues. Demonstration of intracellular bacteria and morula in affected organ tissues can be difficult using special stains (e.g. Romanowsky stain) and confirmatory diagnosis often relies on PCR testing and sequencing.
Natural infections caused by N. risticii have been infrequently reported in cats and dogs with clinical signs (including anorexia, depression, lethargy, fever, vomiting, diarrhea, lymphadenomegaly, weightloss, lameness, polyarthritis) often resembling those of N. risticii infection in horses and N. helmonthica in dogs.7
Contributing Institution:
Department of Veterinary Clinical and Diagnostic Sciences within the Faculty of Veterinary Medicine, University of Calgary.
JPC Diagnoses:
1. Heart: Pancarditis and vasculitis, lymphohistiocytic, multifocal to coalescing, severe, with multifocal myofiber degeneration, necrosis, and loss.
2. Lymph node: Reactive hyperplasia, diffuse, mild to moderate with lymphocytolysis, medullary plasmacytosis, and lymphohistocytic vasculitis.
3. Perinodal fat: Steatitis, lymphohistiocytic, multifocal, mild.
JPC Comment:
"Potomac horse fever" (PHF) was first recognized as a clinical entity in horses in 1979 by veterinarians in Montgomery County, Maryland and Fairfax and Loudon counties, Virginia. Most cases occurred in horses located "within a mile or two" of a six mile long segment of the Potomac River. Between 1982 and 1986, cases dramatically increased in the same region, leading to speculation of a new disease, which was initially known as "Acute
Equine Diarrhea Syndrome" (AEDS). Although first thought to be localized to a focal area adjacent to the Potomac River, PHF has since been recognized in 43 states of the United States, three provinces of Canada, and has been documented in France, the Netherlands, and Brazil.2,10 In addition to PHF, this entity is also known as "equine ehrlichial colitis" (EEC), "equine monocytic ehrlichiosis" (EME), "Shasta River Crud", "churrio", and "churrido equino".2 Interestingly, the term "Potomac horse fever" is credited to a reporter covering the initial outbreak in Maryland, though the term "equine neorickettsiosis" may be more appropriate given its distribution.2,14
In 1984, a rickettsial organism was identified in tissue from a pony experimentally infected with PHF by researchers using transmission electron microscopy. The organism was suggested to belong to genus Ehrlichia given its round shape and location within histiocytic cell vacuoles and was named Ehrlichia risticii. The bacterium was subsequently renamed Neorickettsia risticii in 2001. N. risticii is a gram-negative coccus and stains dark blue to purple with Giemsa and Romanowsky's stains. Serologic diagnosis using indirect fluorescent antibody (IFA) testing is available but can pose a diagnostic challenge for clinicians. Although a 4-fold titer increase is considered diagnostic, antibody levels reach a high point within a few days of infection, requiring collection of serum samples early in the disease process. Previously exposed horses may have a titer of 1:640 for over a year, therefore IFA test titers collected after the initial antibody spike are unable to distinguish between active or past infection, nor vaccination.2 Isolation of R. risticii in cell culture is the gold standard for diagnosis, though this method is both time consuming and often unavailable as a commercial service.1 Confirmatory diagnosis therefore often relies on detection of N. risticii DNA via PCR testing and sequencing as previously noted by the contributor.1,2 A recent report noted feces were the optimal sample utilized for PCR detection, followed by blood.1
PHF typically occurs on a seasonal basis, with the majority of infections occurring during the late summer months, with nearly 70% of the 904 cases reported during the 1982 and 1986 outbreak occurring during July and August. Acute infection in adult horses is typically characterized by of depression, anorexia, and fever followed by moderate to severe diarrhea in approximately 60% of horses that may persist for up to 10 days with a consistency ranging from profuse and watery to "cow-pie-like". Additional clinical signs may include laminitis in 15-30% of PHF cases, subcutaneous edema along the ventral abdomen and limbs, and congested mucous membranes with elevated heart and respiration rates as the result of cardiovascular compromise secondary to both toxemia and dehydration. Overall, the mortality rate of PHF ranges between 17% and 36%, with some cases requiring euthanasia due to secondary complications such as laminitis.2
Experimental cases of PHF are associated with a leukopenia (<5.0 x 109/L) between 8-19 days post infection in up to 90% of horses, followed by a leukocytosis (>14.0 x 109/L) between 13 to 30 days post infection in approximately 50% of horses. Clinical biochemistry results are non-specific.2
Although thought to be a focal entity affecting the eastern United States in 1979, the disease now known as PHF was likely described over half a century earlier by Dr. Frank Schofield in 1924 while investigating a locally endemic disease in Kent and Essex counties of Ontario. The disease was colloquially known by farmers as "cholera", "horse cholera", and "abdominal typhoid". Dr. Schofield described nearly identical clinical signs in horses affected with PHF, which occurred from the middle of July through the beginning of August. Schofield also astutely noted the disease's geographic pattern of distribution, observing, "There is a definite relationship between the water front and the occurrence of disease" and "?one might say that the zone of infection extends from the water front to a distance of about five miles inland". In addition, Schofield noted mayflies (Ephemerida) were found in unusually high numbers in areas with the highest prevalence of disease, stating "?the disease always appears a few days after the May flies appear, and is at its worst by the time the May flies disappear."2
As noted by the contributor, the life cycle of N. risticii is predominantly dependent on trematodes. This infection is thought to be a commensal or mutualistic relationship as infected trematodes do not appear to be negatively affected. In the eastern United States, the maintenance host of N. risticii is Acanthatrium oregonese. This digenic trematode has a complex life cycle, including miracidia and procytes in snail hosts, free-swimming cercariae, metacercariae in aquatic insects (e.g. caddis and mayflies) and egg laying adults that reside in the intestinal lumen of insectivorous bats. N. risticii undergoes vertical transmission with transovarial passage in trematodes. Following a rise in water temperatures, infected snails release N. risticii infected cercaria into the water, which develop into metacercariae, and subsequently infect second intermediate hosts such as fish, amphibians, birds, reptiles, and mammals. In one study using PCR and sequence analysis in an N. risticii endemic region, the pathogen was detected in immature and adult caddisflies (Trichoptera), mayflies (Ephemeroptera), damselflies (Odonata, Zygoptera), dragonflies (Odonata, Anisoptera), and stone flies (Plecoptera) with an overall prevalence of 31.9%.2 Following their emergence, mayflies have been reported to travel as far as 8km inland, which may explain infections of animals lacking access to contaminated rivers and lakes.1
In 1991, a novel species of Neorickettsia also capable of also causing PHF was identified in a horse from Findlay, Ohio and is now known as N. findlayensis. In 2017, this entity was identified in two symptomatic horses in Ontario via culture that were PCR negative for N. risticii. Therefore, N. finlayensis infection may present as a potential confounder when PHF is suspected but PCR testing is negative.1
Additional causes of equine abortion discussed by the moderator included numerous bacterial, mycotic, and viral etiologies in addition to toxins, genetic and nutritional disorders, twinning, umbilical cord torsion, and placental separation.
Both the moderator and conference participants believed the histologic lesions in the submitted tissues were non-specific and definitively diagnosis of equine neorickettsiosis without the use of additional diagnostics, such as PCR in this case, would be challenging. Additional differentials discussed associated with similar lesions in fetal tissue include Neospora sp., Toxoplasma gondii, equine herpes virus-1, equine arteritis virus, and N. risticci. Differentials for similar lesions in adult tissues included Anaplasma phagocytophilium, Lesimania sp., Trypanosoma sp., Neospora sp., equine herpes virus-1, and Toxoplasma gondii.
References:
1. Arroyo LG, Moore A, Bedford S, et al. Potomac horse fever in Ontario: Clinical, geographic, and diagnostic aspects. Can Vet J. 2021;62(6):622-628.
2. Baird JD, Arroyo LG. Historical aspects of Potomac horse fever in Ontario (1924-2010). Can Vet J. 2013;54(6):565-572.
3. Coffman EA, Abd-Eldaim M, Craig LE. Abortion in a Horse Following Neorickettsia Risticii Infection. Journal of Veterinary Diagnostic Investigation. 2008;20: 827-830.
4. Cordes DO, Perry BD, Rikihisa Y, Chickering WR. Enterocolitis caused by Ehrlichia sp. in the horse (Potomac horse fever). Vet Pathol. 1986;23: 471-477.
5. Donahue JM, Williams NM. Emergent Causes of Placentitis and Abortion. Veterinary Clinics of North America: Equine Practice. 2000;16: 443-456.
6. Giles RC, Donahue JM, Hong CB, et al. Causes of abortion, stillbirth, and perinatal death in horses: 3,527 cases (1986-1991). J Am Vet Med Assoc. 1993;203: 1170-1175.
7. Greene CE. Infectious diseases of the dog and cat. St. Louis, Mo.: St. Louis, Mo. : Elsevier/Saunders 4th ed.; 2012.
8. Hong CB, Donahue JM, Giles RC, et al. Equine Abortion and Stillbirth in Central Kentucky during 1988 and 1989 Foaling Seasons. Journal of Veterinary Diagnostic Investigation. 1993;5: 560-566.
9. Long MT, Goetz TE, Whiteley HE, Kakoma I, Lock TE. Identification of Ehrlichia Risticii as the Causative Agent of two Equine Abortions Following Natural Maternal Infection. Journal of Veterinary Diagnostic Investigation. 1995;7: 201-205.
10. Paulino PG, Almosny N, Oliveira R, et al. Detection of Neorickettsia risticii, the agent of Potomac horse fever, in horses from Rio de Janeiro, Brazil [published correction appears in Sci Rep. 2020 Jul 29;10(1):13001]. Sci Rep. 2020;10(1):7208. Published 2020 Apr 29.
11. Pusterla N, Madigan JE, Chae J-S, DeRock E, Johnson E, Pusterla JB. Helminthic Transmission and Isolation of Ehrlichia risticii, the Causative Agent of Potomac Horse Fever, by Using Trematode Stages from Freshwater Stream Snails. Journal of Clinical Microbiology. 2000;38: 1293-1297.
12. Pusterlaa N, Leuteneggera CM, Sigristb B, Chaea J, Lutzb H, Madigana JE: Detection and quantitation of Ehrlichia risticii genomic DNA in infected horses and snails by real-time PCR. 2000
13. Teymournejad O, Lin M, Bekebrede H, et al. Isolation and Molecular Analysis of a Novel Neorickettsia Species That Causes Potomac Horse Fever. mBio. 2020;11.
14. Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier; 2016:200.
15. Williams NM: Disorders of Horses, pp. 147-171. Oxford, UK: Wiley‐Blackwell, Oxford, UK, 2012