Wednesday Slide Conference, Conference 16, Case 4
Signalment:
5-year-old, female, Scottish blackface sheep (Ovis aries).
History:
Presented to the Veterinary School farm animal clinic for investigation of ill thrift. Treatment was given in response to a high faecal parasite egg count and the numbers decreased. The submitting farm has a history of ovine pulmonary adenocarcinoma.
Gross Pathology:
Within the abdomen approximately 1 litre of yellow-tinged clear, watery fluid (hydroperitoneum) is noted.
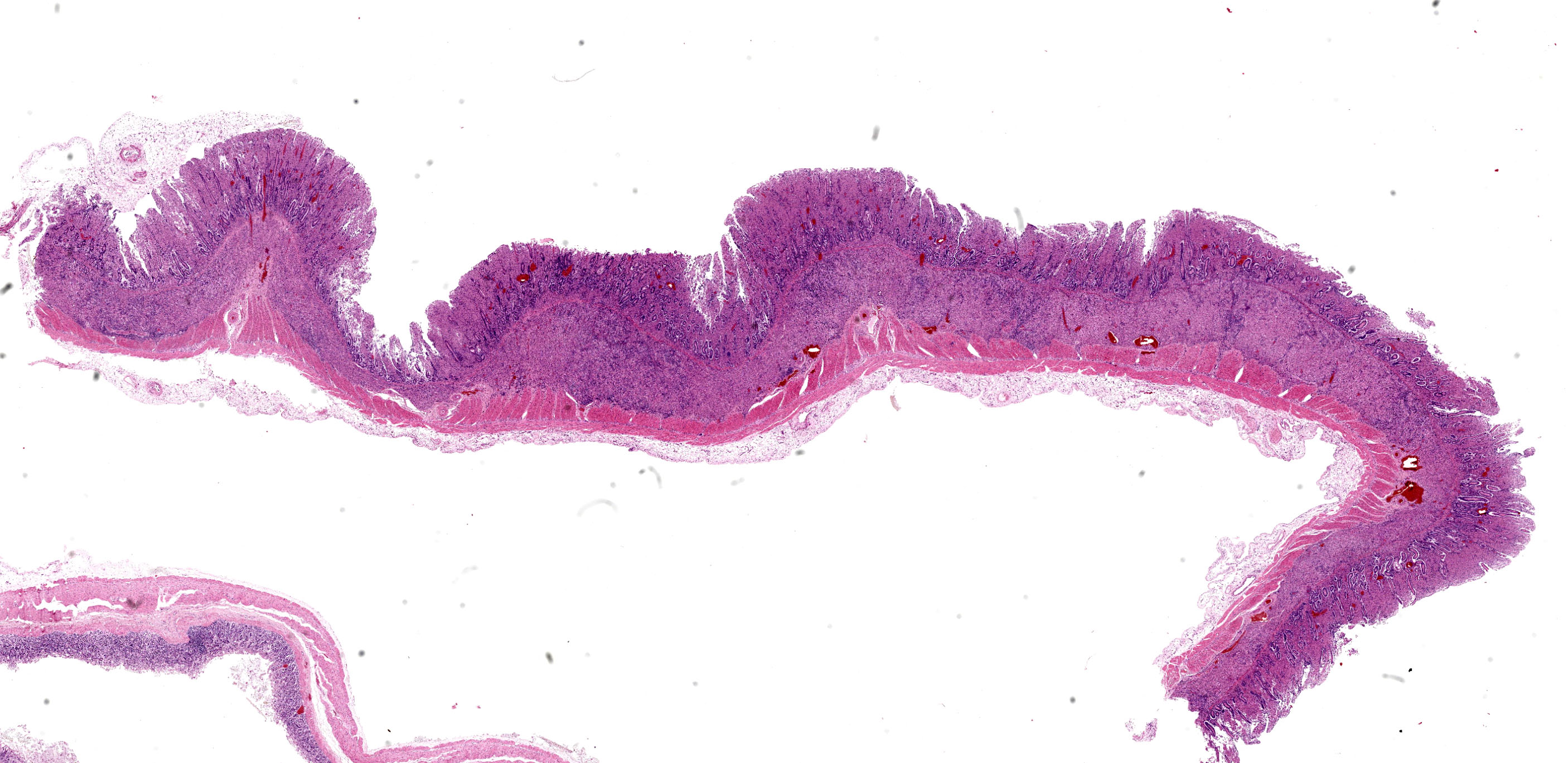
Diffusely, the walls of the ileum and the distal third of the jejunum are moderately to markedly thickened, the mucosal surface exhibits a corrugated pattern and is bright yellow in colour. The mucosa of the caecum, diffusely, and colon, segmentally, are mildly to moderately thickened.
Multifocally, the mesenteric lymph nodes are moderately enlarged, and upon cut section show moderate multifocal expansion of the cortex by firm white-grey tissue.
Laboratory Results:
Parasitology:
- McMaster slide method: 2000 strongyle spp. eggs per gram (epg), 500 Moniezia spp. epg, 1100 Coccidia spp. epg, 100 Nematodirus spp. epg
- Baermann technique: positive for Dictyocaulus filaria larvae.
Serology:
- Corynebacterium pseudotuberculosis: Negative
- Maedi-Visna virus: Negative
- Mycobacterium avium subsp. Paratuberculosis (MAP): Positive
Microscopic Description:
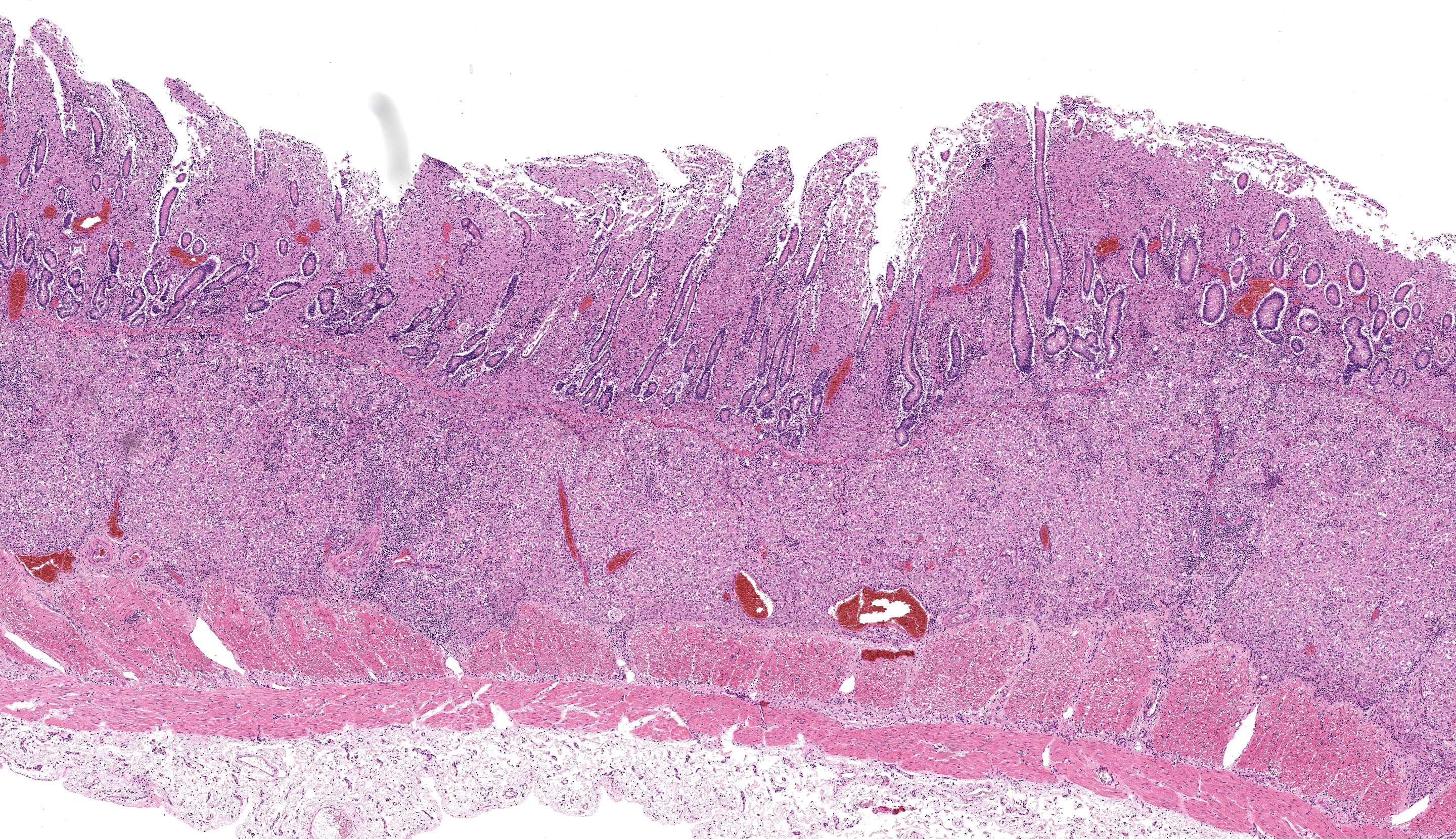
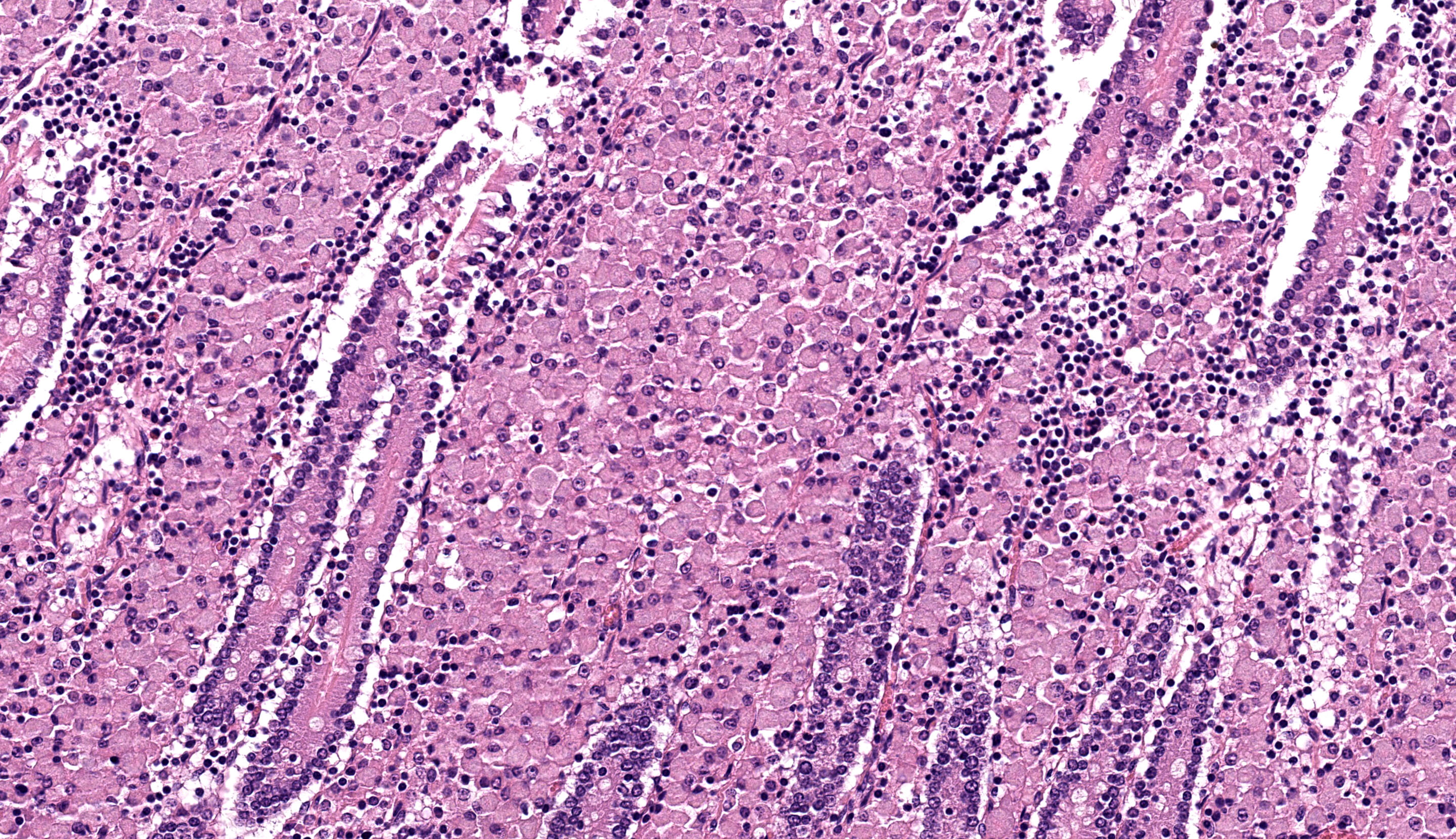
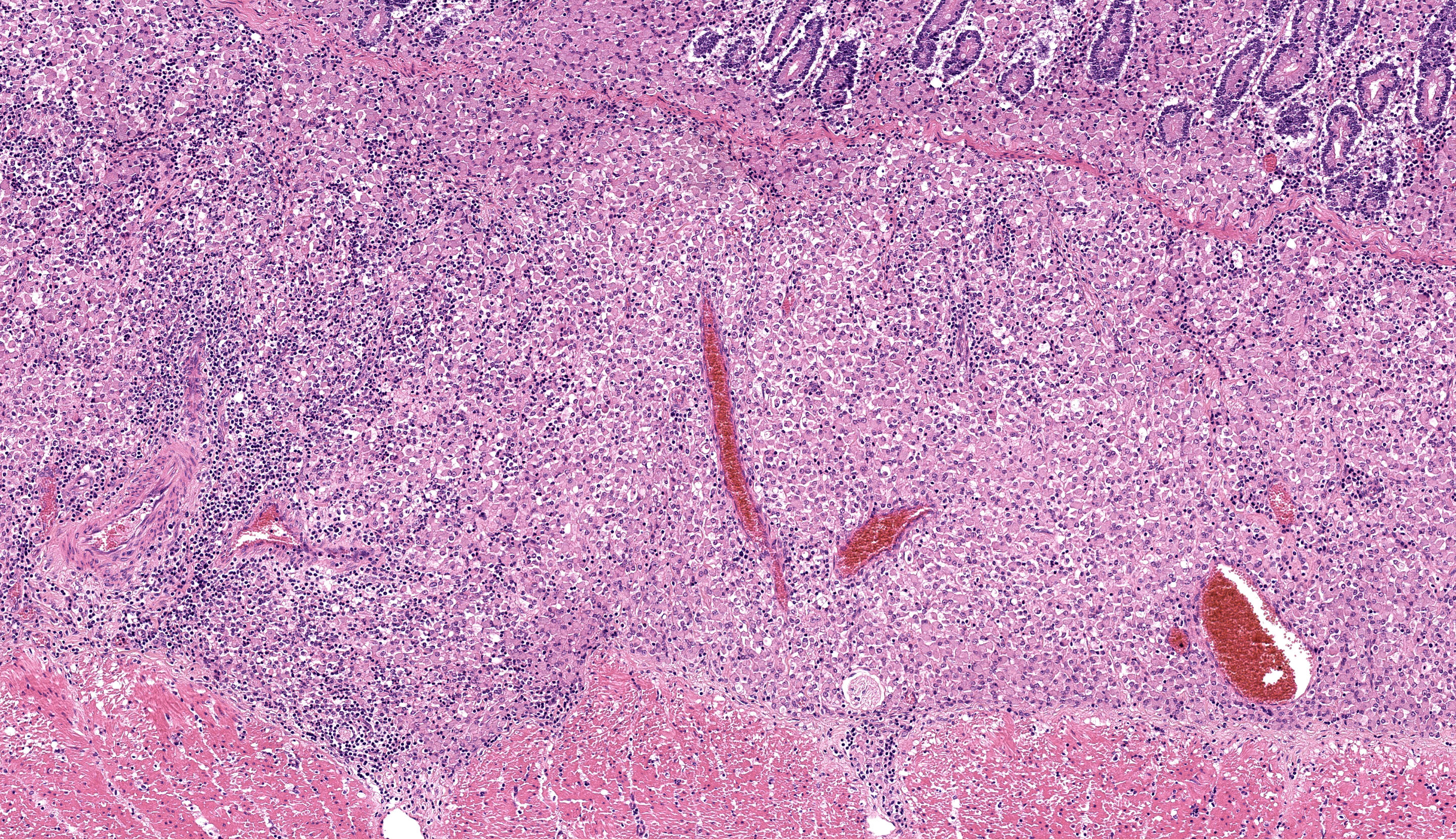
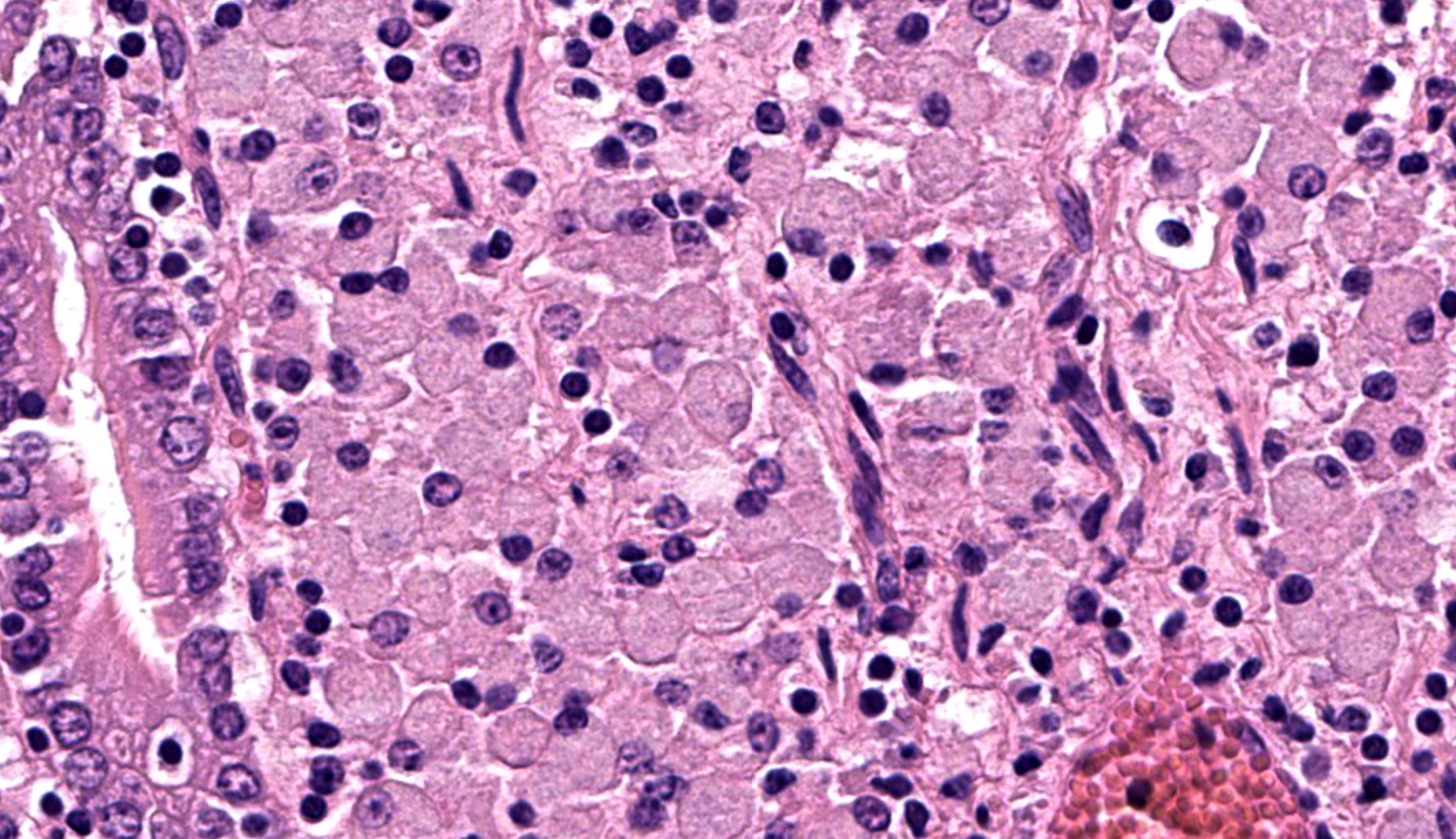
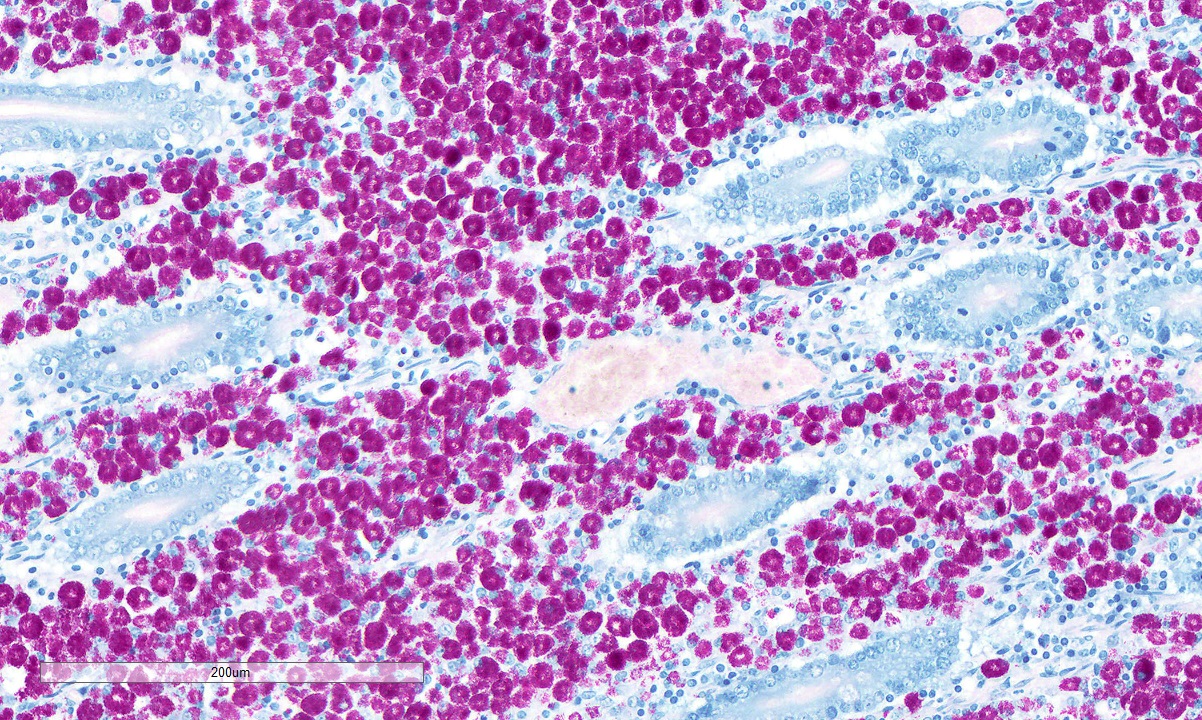
Caecum: Separating intestinal crypts, the lamina propria and submucosa are diffusely markedly expanded by large numbers of epithelioid macrophages. Within the cytoplasm of these cells there are large numbers of 4x1µm bacilli that fail to stain with HE stain but are acid-fast positive on Ziehl-Neelsen stain. Admixed with the macrophages are small numbers of lymphocytes, plasma cells and occasional multinucleate giant cells. Similar inflammatory cell infiltrates extend, multifocally, into the inner circular and outer longitudinal muscular layers of the caecal wall. The serosa is expanded, diffusely, and the connective tissue is separated by clear, material (protein poor oedema) and contains small numbers of lymphocytes, plasma cells and macrophages, that latter containing acid-fast bacilli, which are present rarely in presumed lymphatics. Small numbers of intestinal crypts are lined by flattened enterocytes. The blood vessels are filled with moderate numbers of erythrocytes (congestion).
Contributor’s Morphologic Diagnosis:
Caecum: Typhlitis, chronic, histiocytic, diffuse, marked with intra-histiocytic acid-fast bacteria.
Contributor’s Comment:
The microscopic features of this enteritis are consistent with diffuse multibacillary Mycobacterium avium subsp. paratuberculosis (MAP) infection, also known as Johne’s disease.3,7,12
MAP is a Gram-positive, acid-fast, aerobic, non-motile, non-spore forming bacterium of the genus Mycobacterium, family Mycobacteriacea and a member of the Mycobacterium avium complex which comprises M. avium avium, M. avium sylvaticum, M. colombiense and M. intracellulare.10
Premortem diagnosis is challenging, and the gold standard is based on faecal culture, which takes several weeks to months,3 or other tests such as Ziehl-Neelsen stained faecal smears, ELISA, PCR, identification of MAP specific peptides, and expression of gamma interferon all of which will provide more rapid results. Liver biopsy has also been shown to have high sensitivity and specificity for identifying sheep with advanced disseminated infection.9 On post-mortem examination, the gold standard is based on culture and isolation of the bacteria although PCR of affected intestinal tissue samples for Insertion Sequence 900 (IS900) provides faster results and is MAP specific.3,6,11
Infection is thought to occur in neonates and juveniles via ingestion of contaminated food, pasture, water, colostrum, and milk. MAP is thought to cross the intestinal mucosal barrier via preferential uptake by M-cells present overlying Peyer’s patches and mucosal lymphoid domes. Bacteria then invade subepithelial macrophages and modulate the immune system3,10,12 whilst concurrently inhibiting phagolysosome fusion, acidification and macrophage activation to avoid degradation.4 Typically, the infection takes months to years before causing macroscopic lesions and clinical signs. Experimental challenge in sheep usually requires an incubation period of 4 to 8 months before the onset of clinical signs. Animals infected before 2 years of age exhibit greater pathological and clinical manifestations compared to those infected when adult.
Depending on the ensuing immune response to infection, different morphological presentations of disease develop. The paucibacillary form of the disease is driven by a Th1 cell-mediated interferon gamma response whereas the multibacillary Th2 form is a humoral response driven by IL-10. The multibacillary morphology is thought to be caused by a switch from a Th1 response towards a Th2 response or may be caused by a simultaneous Th1 and Th2 response with failure of the Th1 component of inflammation.7 Depending on the inflammatory response, the affected macrophages display different phenotypes, either M1 iNOS (inducible nitric oxide synthase) and TNF-alpha positive activated macrophages driven by Th1 mediated inflammation or M2 CD163, IL-10 and TGF-beta positive activated macrophages mediated by a Th2 response.2 Additionally, 3 different subsets of M2 macrophages have been described: M2a is present in allergies and helps with killing and encapsulating parasites, M2b is driven by a Th2 inflammatory response induced by IL-1 and M2c is induced by IL-10 and TGF-beta and is believed to be implicated in Johne’s disease progression towards the multibacillary form.2
MAP is subdivided in type I/III and type II strains which affect, predominantly, sheep and cattle, respectively. Additionally, types II and III have broader host ranges.6,11 MAP has been reported in numerous species including a wide range of captive and wild ruminants, camelids, rabbits, equids, swine, lagomorphs and primates. It has also been detected in rodents, carnivores and several species of wild birds but without evidence of disease.11 Rabbits may play a role in the epidemiology of the disease although it is not clear if they are naturally susceptible to the disease.1 Furthermore, MAP has been isolated from humans suffering from Crohn’s disease by PCR and FISH, but in human tissues it is in a mycobacterial cell wall deficient state, so is not acid-fast.6,11
Contributing Institution:
Veterinary Diagnostic Services
School of Biodiversity, One Health & Veterinary Medicine
College of Medicine, Veterinary and Life Sciences
University of Glasgow
https://www.gla.ac.uk/schools/vet/cad/
JPC Diagnosis:
Intestine: Enteritis, granulomatous, chronic, diffuse, severe, with edema.
JPC Comment:
The conference concludes with a classic entity that has been a frequent submission to the WSC. There was spirited debate on tissue identification in this case as participants were divided on whether or not there were villi present (i.e. consistent with small intestine) – Dr. Uzal was unable to resolve this question definitively, so we hedged with the lessspecific “intestine” in our morphologic diagnosis.
The microscopic features of this case align with the Th2 multibacillary pathogenesis that the contributor nicely summarizes. Sheets of macrophages expand the mucosa, lamina propria, and submucosa and ultimately result in a malabsorptive diarrhea that corresponds to the ill thrift noted clinically for this ewe. Dr. Uzal discussed that the submucosal involvement in this case was not unusual for MAP, though it may be overlooked in the description.
One feature not reported in this case is mineralization of the aorta and endocardium which is reported to occur in up to 25% of paratuberculosis cases.8 Macrophage activation, particularly of M1 macrophages, likely drives calcification of elastic fibers via deposition of vitamin D metabolites in response to cytokines such as TNF-α, IL-1, and IL-8.6 Plasticity of vascular smooth muscle to differentiate towards an osteoblastic phenotype under the influence of this same cytokine milieu likely also plays a role in vascular mineral deposition.6 Conversely, M2 macrophages impair osteogenesis and/or enhance osteolysis and mineral degradation. The interplay of secreted matrix metalloproteases, available matrix constituents, and concurrent injury to the vessel via reactive oxygen species (ROS) has also been evaluated.6 Although not as well studied as in human atherosclerosis in which these processes have been more extensively studied, an understanding of this basic process is helpful for understanding mineralization in other diseases. Important ruleouts in this case include ingestion of calcinogenic plants (e.g. Cestrum diurnum and Trisetum flavescens) and hypervitaminosis D.
References:
- Arrazuria R, et al. Mycobacterial Infections in Rabbits: From the Wild to the Laboratory. Transbound Emerg Dis. 2017; 64(4):1045-1058.
- Fernández M, et al. Macrophage Subsets Within Granulomatous Intestinal Lesions in Bovine Paratuberculosis. Vet Pathol. 2017; 54(1):82-93.
- Idris SM, et al. Paratuberculosis: The Hidden Killer of Small Ruminants. Animals (Basel). 2021; 12(1):12.
- Jenvey CJ, et al. Quantification of Macrophages and Mycobacterium avium Subsp. paratuberculosis in Bovine Intestinal Tissue During Different Stages of Johne's Disease. Vet Pathol. 2019; 56(5):671-680.
- Li Y, Sun Z, Zhang L, Yan J, Shao C, Jing L, Li L, Wang Z. Role of Macrophages in the Progression and Regression of Vascular Calcification. Front Pharmacol. 2020 May 8;11:661.
- Liverani E, et al. Mycobacterium avium subspecies paratuberculosis in the etiology of Crohn's disease, cause or epiphenomenon? World J Gastroenterol. 2014; 20(36):13060-13070.
- Marquetoux N, et al. A synthesis of the patho-physiology of Mycobacterium avium subspecies paratuberculosis infection in sheep to inform mathematical modelling of ovine paratuberculosis. Vet Res. 2018; 49(1):27.
- Rosa FB, Roussey J, Coussens PM, Langohr IM. Pathology in practice. Johne's disease. J Am Vet Med Assoc. 2013 Jun 15;242(12):1655-7.
- Smith SL, et al. Liver biopsy histopathology for diagnosis of Johne's disease in sheep. Vet Pathol. 2014; 51(5):915-918.
- Ssekitoleko J, et al. Mycobacterium avium subsp. paratuberculosis Virulence: A Review. Microorganisms. 2021; 9(12):2623.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier; 2016:194-197.
- Windsor PA. Paratuberculosis in sheep and goats. Vet Microbiol. 2015; 181(1-2):161-169.