Wednesday Slide Conference, 2025-2026, Conference 4, Case 1
Signalment:
Unknown age, male, black-tailed prairie dog, Cynomys ludovicianusHistory:
Prairie dog sourced from a zoologic collection. Clinical presentation for acute ptyalism, easily captured, poor vision. Clinical examination noted superficial healing wounds on bilateral forearms, facial scarring with intermittent swellings, incisor malocclusion, elongate buccal aspects of mandibular molars, bilateral tongue ulceration.Gross Pathology:
External examination of the lungs was normal. On serial sectioning and trimming in representative sections of lung a single small sample of lung tissue was noted as floating low in formalin solution; on examination of one aspect of the cut section the lung was pale tan and consolidated with multifocal pale tan, circular, soft foci (exudate filling bronchi). The total volume of affected lung mass was less than 10%.Laboratory Results:
Initial Gram, GMS, and Acid-Fast stains of affected lung tissue did not identify bacterial or fungal microorganisms. Please see comments below for details on follow-up ancillary diagnostics.Microscopic Description:
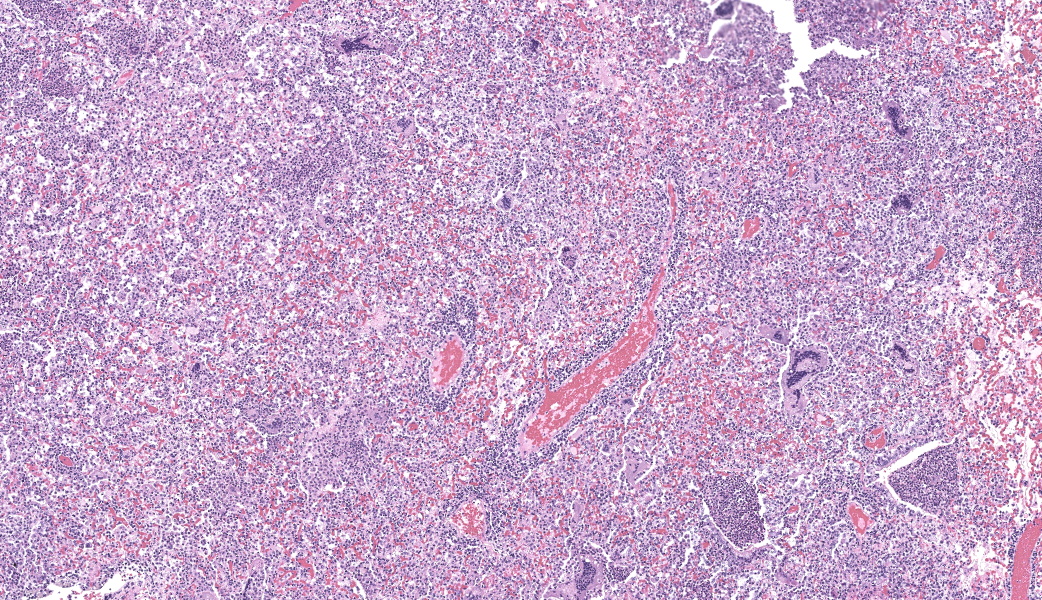
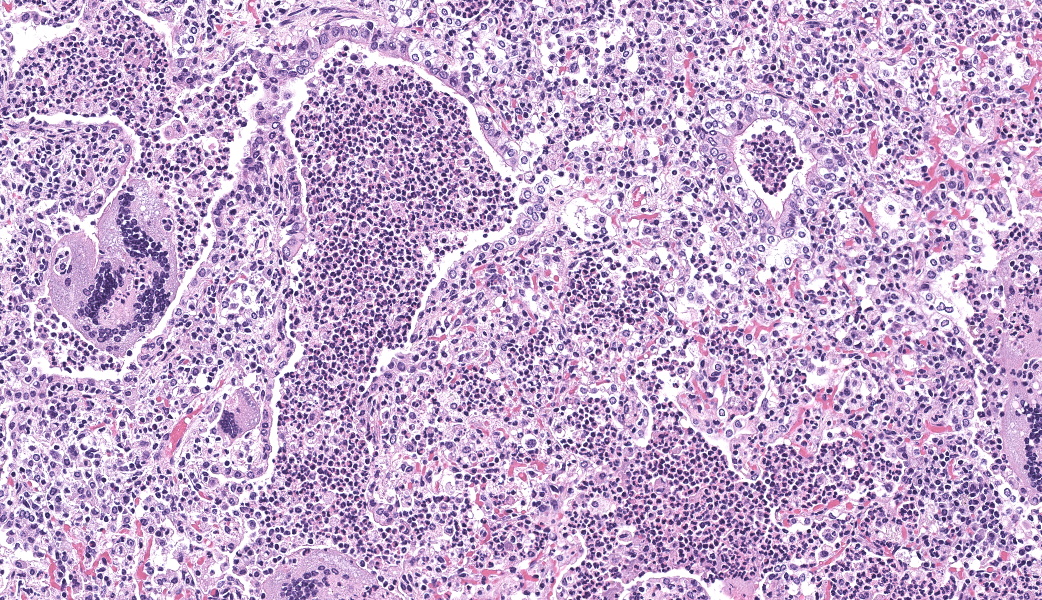
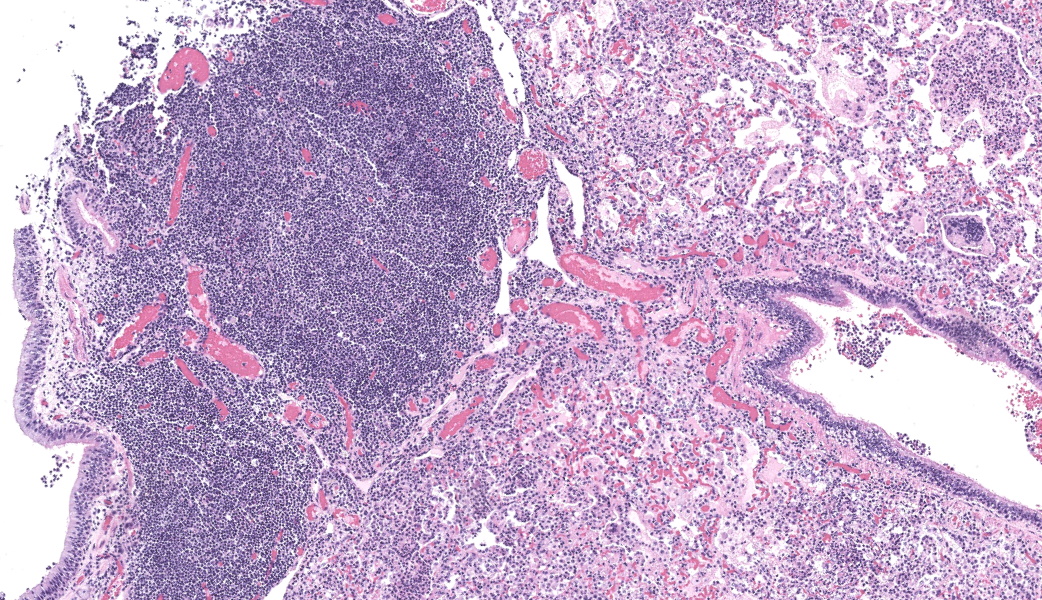
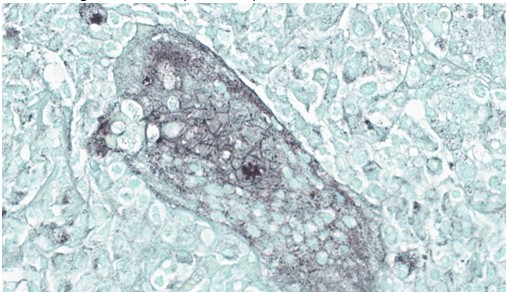
Lung, multiple sections. Within a single section of lung, approximately 75% of the bronchial, bronchiolar, and alveolar lumina contain overall large numbers of neutrophils, macrophages, large multinucleated cells, cellular debris, homogenous eosinophilic material, fibrillary eosinophilic material, and occasionally scattered erythrocytes. The multinucleated cells are often large and bizarre in appearance, some containing approximately 40 nuclei. The cytoplasm of these multinucleated cells is eosinophilic and variably granular to homogenous. A subset of these multinucleated cells contains few distinct optically clear vacuoles, while others demonstrate phagocytosis of few neutrophils. There is mild vascular congestion throughout, and there are small clusters of lymphocytes cuffing airways multifocally.Contributor's Morphologic Diagnoses:
Pneumonia, bronchoalveolar, suppurative, histiocytic, focal, subacute, severe with intraluminal multinucleated giant cells.Contributor's Comment:
Initial Gram, GMS, and acid-fast stains of affected lung tissue did not identify bacterial or fungal microorganisms. Viral inclusions were not evident on H&E-stained sections of the affected lung and other tissues evaluated during necropsy evaluation.Pathologists reviewing the case considered the possibility of multinucleated giant cells versus syncytia. Pulmonary syncytia in mammalian species may be seen with orthopox viruses, herpes viruses, and respiratory syncytial viruses.5 Among the orthopox viruses, monkeypox virus would be a possible differential that must be considered given this species’ vulnerability to the virus and the zoonotic potential.2,7 Necrotizing bronchopneumonia has been reported in prairie dogs with monkeypox viral infection, and histologic descriptions have included syncytia and poorly discernible eosinophilic intracytoplasmic inclusions.4,6
Formalin-fixed, paraffin-embedded lung tissue was submitted to the Center for Disease Control and Prevention (CDC) for further evaluation. Immunohistochemical staining for orthopoxviruses was not detected. Additional immunohistochemical stains for canine distemper virus, measles virus, and respiratory syncytial virus were reported negative. PCR testing for human parainfluenza virus (1-4) and respiratory syncytial virus were also reported negative.
Transmission electron microscopy provided ultrastructural evidence of bacteria within giant cells, with no viral particles identified.
The sample was immunoreactive on a polybacterial immunohistochemical assay, but negative for select gram-negative bacteria on a separate immunohistochemical assay. PCR testing for the groEL gene of mycobacteria and Nocardia spp. was positive, with subsequent sequencing identifying Nocardia spp. PCR testing for gram-negative bacteria specific 16S rRNA gene was positive for Pasteurellaceae. Immunohistochemical staining for Pasteurella multocida was negative. PCR testing for gram-positive bacteria specific 16S rRNA gene was indeterminate. Repeat acid fast staining (Fite’s stain) identified long filamentous rods within the giant cells.
Contributing Institution:
Arizona Veterinary Diagnostic Laboratory, https://azvdl.arizona.eduJPC Diagnoses:
Lung: Bronchopneumonia, histiocytic and neutrophilic, chronic, multifocal to coalescing, severe, with numerous multinucleated giant cells.JPC Comment:
Our fourth conference this year was moderated by the esteemed Dr. Thomas Cecere from Virgina Tech. The JPC team was thrilled to have him back for the second year in a row. This first case provided a great discussion on diagnostic workups and the processes that the pathologist should consider when choosing next steps in a case. Here, the lungs were “chock-a-block” full with histiocytic and neutrophilic inflammation with numerous giant cells that one participant remarked as having “a million nuclei.” These were some of the most impressive giant cells that many participants had seen. The bacteria were very difficult to see on the H&E, but the pattern of inflammation and presence of such robust giant cells should clue one into the presence of infectious organisms. As such, next steps should include a full gamut of routine infectious organism stains, including gram stains, acid fast stains, and fungal stains (GMS, PAS). Given that these were performed by the contributor, only confirmatory GMS and Fite-Faraco (FF) stains were performed in house, which revealed weakly acid-fast, GMS-positive organisms within multinucleated giant cells. This is consistent with the Nocardia spp. that were identified by the contributor. Those “chef’s kiss” multinucleated giant cells, though, deserve some recognition here.The multinucleated giant cell macrophage is a truly remarkable physiologic phenomenon. How they are formed is poorly understood. The current understanding is that macrophages need to be present in chronic inflammation, where they are constantly exposed to pro-inflammatory cytokines, such as IFN-γ, IL-3, IL-4, IL-13, and GM-CSF, as well as pathogen-associated molecular patterns (PAMPs) and other mediators of inflammation.1 A common setting in which these “ingredients” are found is in fungal infections or when dealing with foreign bodies, both of which can sometimes be too large for inflammatory cells to phagocytose and handle on their own. In this environment, macrophages will be in close association with one another and will begin to express molecules on their cell surface that enable fusion with one another, including dendritic cell-specific transmembrane protein (DC-STAMP; major driver of fusion), β1 and β2 integrins, CD44 (hyaluronic acid receptor), CD47 (integrin-associated protein), macrophage fusion protein receptor (MFPR), fusion regulatory protein (FRP-1, also known as CD98), and P2X7 (an ATP-activated ion channel that results in pore formation).1
Now that the involved macrophages have fused together into their version of Infinity Man, they are better equipped with their combined powers to engulf larger threats. One of the mechanisms that multinucleated giant cells have at their disposal to combat pathogens is to produce abundant amounts of nitric oxide (NO), which is generally microbicidal via induced oxidative stress.3 However, Mycobacterium spp. and Nocardia spp. target macrophages as their preferred home within the body and are able to survive intracellularly by preventing fusion of the phagosome and lysosome. Additionally, they produce enzymes to help them combat the damaging effects of NO and other reactive oxygen species produced by macrophages. Multinucleated giant cells are, for all their pathogen-fighting abilities, rather permissive to the persistence of Mycobacterium spp. and Nocardia spp. within them.3 With multinucleated giant cells pumping out a ton of NO, other macrophages are additionally induced to fuse and become multinucleated giant cells, which further perpetuates Mycobacterial survival and can contribute to the chronic nature of Mycobacterium spp. and/or Nocardia spp. infections.3
References:
- Ackermann MR. Inflammation and Healing. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 7th ed. St. Louis, MO: Elsevier; 2022:104-170.
- Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Micro. 2010;140:229-236.
- Gharun K, Senges J, Seidl M, Lösslein A, Kolter J, Lohrmann F, Fliegauf M, Elgizouli M, Alber M, Vavra M, Schachtrup K, Illert AL, Gilleron M, Kirschning CJ, Triantafyllopoulou A, Henneke P. Mycobacteria exploit nitric oxide-induced transformation of macrophages into permissive giant cells. EMBO Rep. 2017;18(12):2144-2159.
- Guarner J, Johnson BJ, Paddock CD, Shieh WJ, et al. Monkeypox transmission and pathogenesis in prairie dogs. Emerg Inf Dis. 2004;10(3):426-431.
- Hughes L, Olson VA, Damon IK. Poxviruses. In: Jorgensen JH, ed. Manual of Clinical Micrology. 11th ed. ASM Press; 2015.
- Langohr IM, Stevenson GW, Thacker HL, Regnery RL. Extensive lesions of monkeypox in a prairie dog (Cynomys sp.). Vet Pathol. 2004;41(6):702-707.
- Yarto-Jaramillo E. Rodentia. In: Miller RE ed. Fowler’s Zoo and Wild Animal Medicine. Vol 8. Saunders; 2015.