CASE 4: 50365 (4153012-00)
Signalment:
7-year-old, female spayed, domestic shorthair cat (Felis catus)
History:
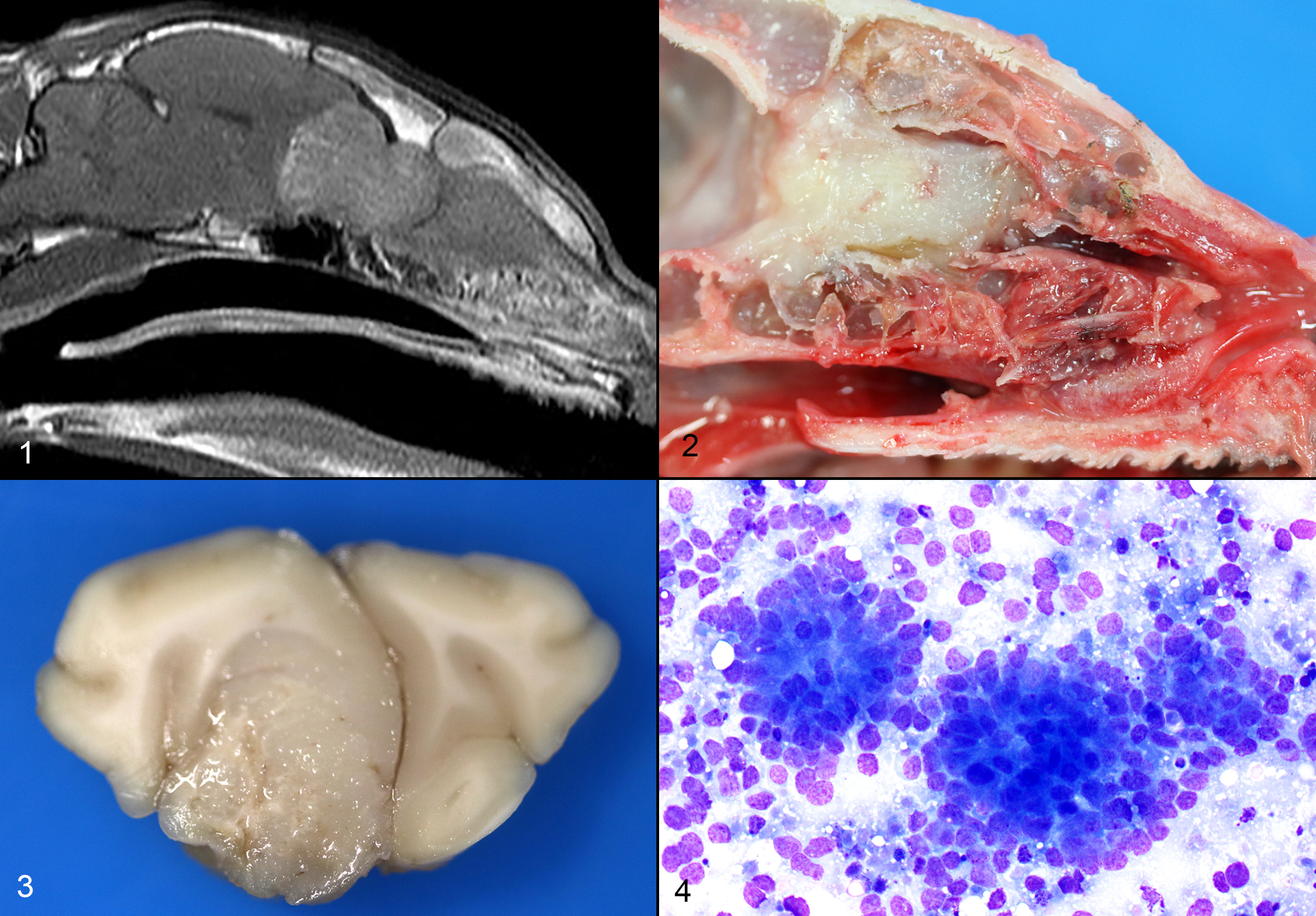
The cat presented with a several day history of inappetence and progressive tetra-ataxia. Supportive treatment was initiated. An evaluation by the Neurology department was suggestive of left central vestibular disease. An MRI was obtained, which showed an expansile soft tissue mass extending from the nasal cavity, through the cribriform plate and into the frontal sinuses and brain. A marked mass effect with trans-tentorial and foramen magnum brain herniation was observed.
Gross Pathology:
Respiratory: The nasal cavity has a white, firm, gelatinous mass that measures 2 x 1 x 1 cm and is most prominent on the left side. Impression smears are obtained.
Nervous: The left frontal lobe of the brain has a focal, firm, white, gelatinous mass communicating with the nasal cavity that measures approximately 1.5 x 1.0 x 1.0 cm. The regional gyri are expanded (edema). The cerebellar vermis is markedly flattened and is visible through the foramen magnum. Upon removal of the calvarium, the vermis is markedly compressed and elongate (cerebellar coning; transforaminal herniation). Mild indentation of the occipital lobes is observed (presumed trans-tentorial herniation).
Laboratory results:
Three impression smears of the left frontal lobe/nasal mass are examined. All samples are highly cellular and contain clusters of cells forming occasional acinar-like structures upon a background of amphophilic to basophilic (mucinous) material and peripheral blood. The cells are round to oval, with mild to moderate pleomorphism, and moderate amounts of basophilic cytoplasm. Many cells do not contain cytoplasm. Nuclei contain prominent single or multiple nucleoli. Occasional mitoses are found.
Microscopic description:
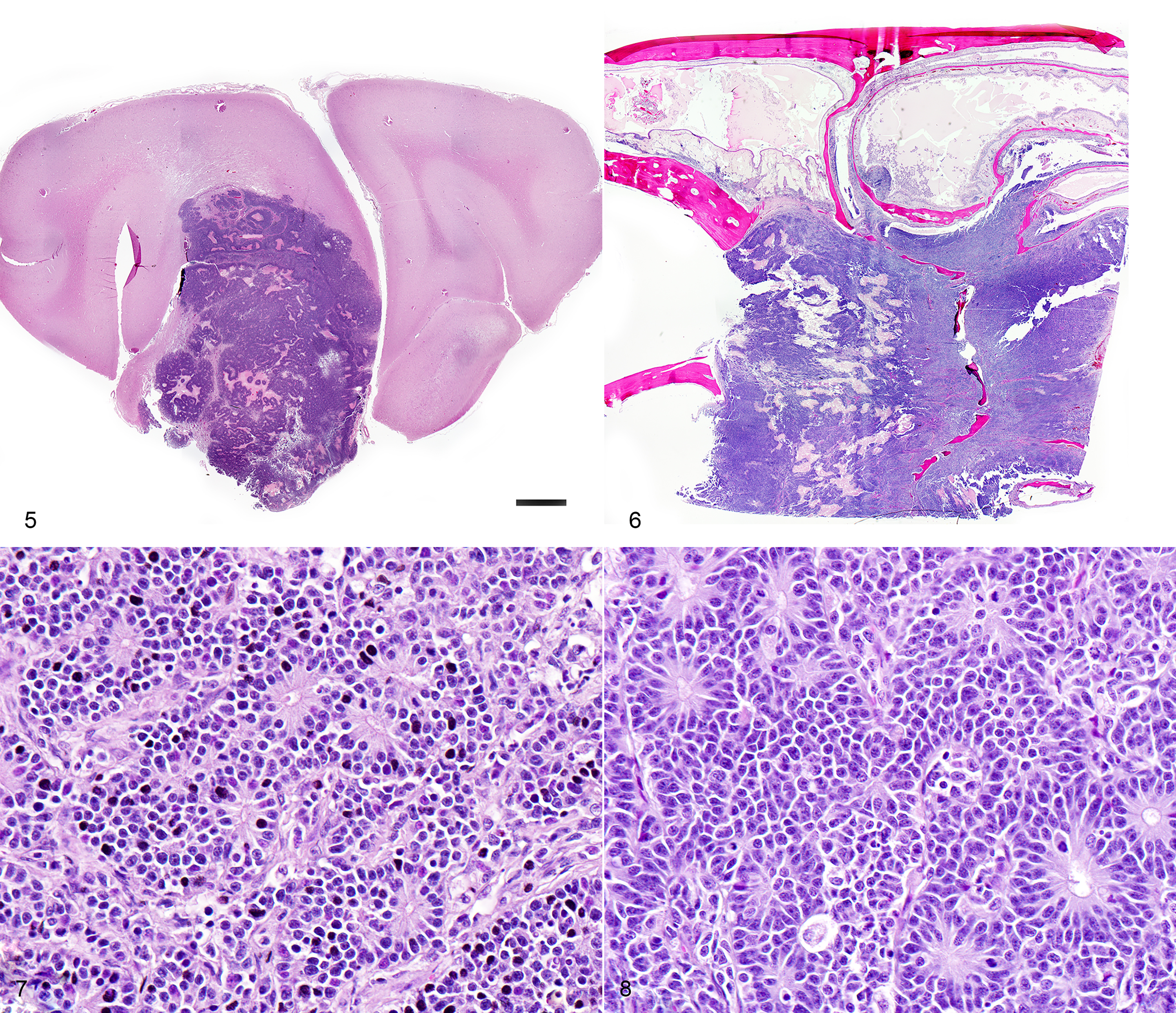
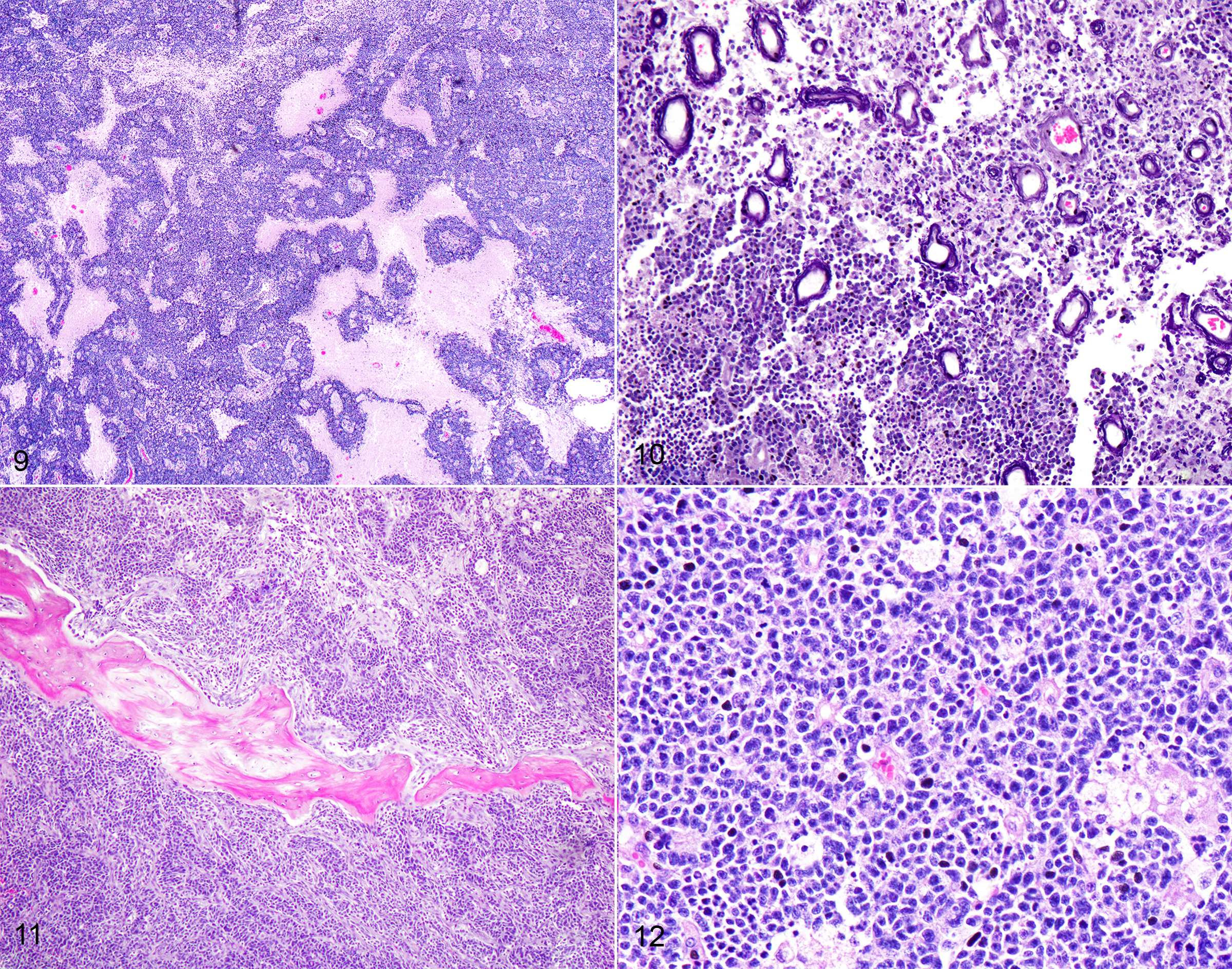
One section each from the rostral brain and nasal cavity contains a similar neoplastic process. The masses are well demarcated but infiltrative, densely cellular and comprised of neoplastic cells forming sheets, acini, rosettes and pseudorosettes amongst a scant fibrovascular stroma. The rosettes in some cases have a distinct lumen (Flexner-Wintersteiner); others contain fibrillar cytoplasmic processes (Homer-Wright) (brain), (nasal cavity). Less frequent perivascular pseudorosettes are observed. Neoplastic cells are round, oval and cuboidal to columnar, with scant to moderate amounts of eosinophilic cytoplasm and round to oval central or basilar nuclei. Nuclei contain moderately coarse chromatin and an indistinct or single central nucleolus. In regions with the highest mitotic activity, there are greater than 100 mitoses in an area of 2.37mm2 (10 high power fields with a 40x objective, 10x ocular FN 22mm, FOV diameter 0.55 mm).3 Multifocal to coalescing necrosis is scattered throughout the neoplastic populations in both locations, with regional nuclear pyknosis and karyorrhectic debris. Mineralized vessels are observed in the brain sections. In the adjacent brain parenchyma, there are variable amounts of rarefaction with increased numbers of glial cells, including gemistocytic astrocytes and Gitter cells. In these regions, small caliber vessels are prominent with increased branching, endothelial hypertrophy and loosely separated white matter (edema). Few scattered perivascular lymphocytes and plasma cells are observed. In the leptomeninges of the surrounding brain, small populations of inflammatory cells are present, including lymphocytes and plasma cells.
In the nasal cavity, inflammatory cells are present adjacent to the mass, including numerous neutrophils with fewer foamy macrophages, lymphocytes, plasma cells and occasional lymphoid follicle formation. The turbinate bones are irregular with reversal lines and multifocal lysis with infiltration by the neoplastic populations.
Contributor's morphologic diagnosis:
Brain and nasal cavity: Olfactory neuroblastoma (cannot rule out neuroendocrine carcinoma) with intralesional and regional necrosis, edema, gliosis, encephalitis? and rhinitis, neutrophilic, lymphoplasmacytic, moderate, chronic.
Mitotic count: Up to 115
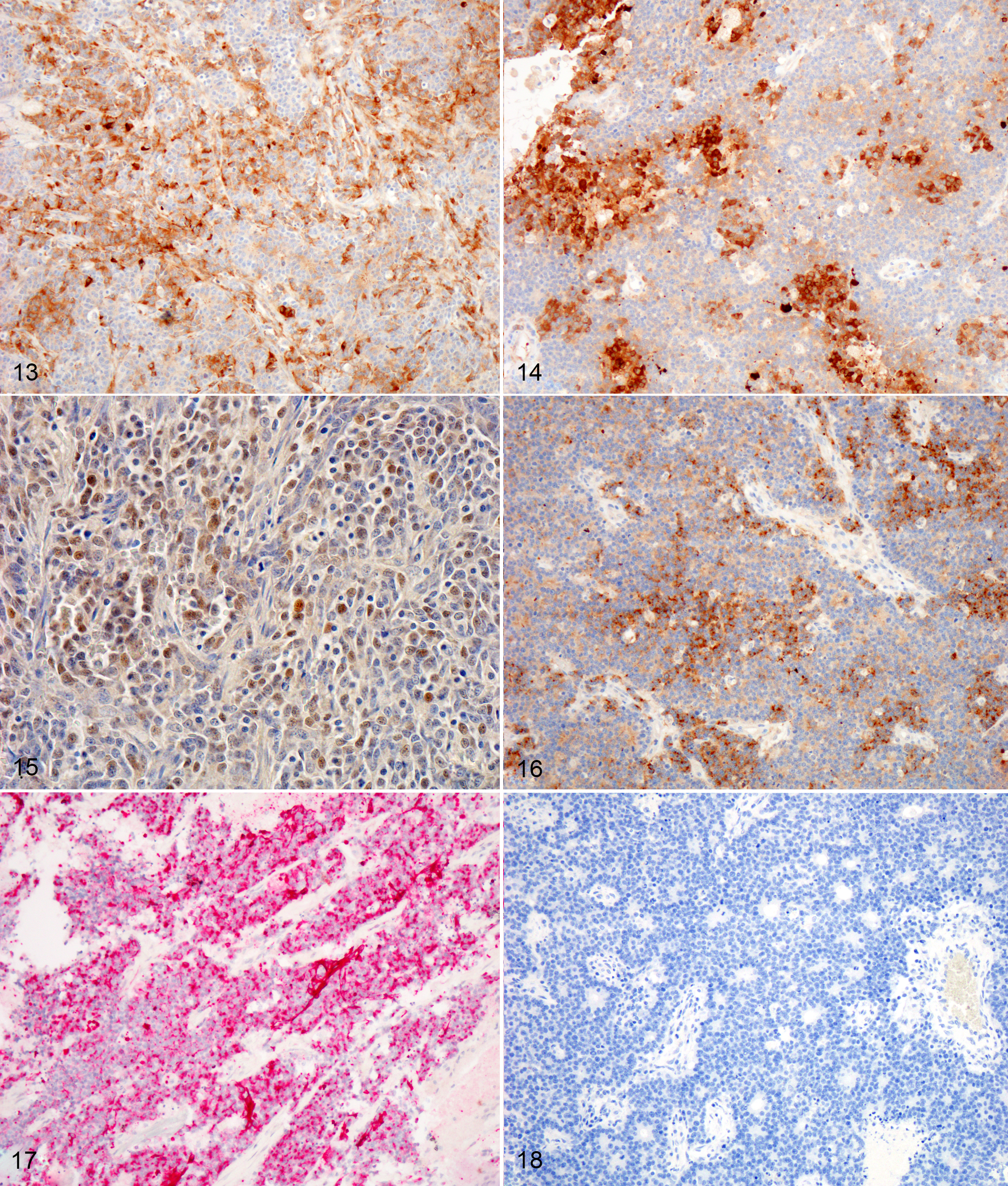
Immunohistochemistry (brain and nasal cavity): Immunopositive for MAP-2, pancytokeratin (AE1/AE3), Synaptophysin, NSE; scant immunopositivity for NeuN, immunonegative for Chromogranin
Contributor's comment:
The histologic and immunohistochemical findings are supportive of a primary olfactory neuroblastoma (esthesioneuroblastoma), however, neuroendocrine carcinoma cannot be ruled out without ultrastructural evaluation or additional immunohistochemistry. This neoplasm infiltrated through the cribriform plate and into the brain, resulting in increased intracranial pressure, transforaminal and transtentorial herniation. In one case series, the most common clinical signs in dogs and cats with olfactory neuroblastoma (ONB) included unilateral epistaxis, nasal discharge, anorexia, aggression and central nervous system (CNS) dysfunction.1 Additional differential diagnoses include neuroendocrine carcinoma, sinonasal undifferentiated carcinoma and malignant melanoma.1,12
Tumors of the nasal cavity and sinuses are rare to infrequent in cats.1,5,12 Up to 90% of feline nasal and paranasal sinus tumors are reported to be malignant.7 Olfactory neuroblastoma (ONB) is a rare malignant neuroectodermal tumor of the nasal cavity and paranasal sinuses, thought to be derived from the olfactory neuroepithelium.1,5,12 Olfactory neuroepithelium is present in the dorso-caudal nasal cavity and includes the rostral turbinates, cribriform plate and rostral nasal septum.5,7 Indirect evidence suggests that these tumors may originate from olfactory stem cells.5,8 These tumors are typically locally invasive with osteolysis and ethmoturbinate deformation.9 Although the imaging features of this neoplasm are nonspecific, identification of a mass in the caudal nasal cavity with extension into the neurocranium are features that generate increased suspicion for ONB.10 Metastasis to regional lymph nodes and ocular structures has been reported in domestic animals, with one case report of distant metastasis to the liver and lungs in an Afghan hound.9 There was no evidence of metastasis in the regional lymph nodes or lungs in this case.
The histologic appearance of ONBs is varied with a spectrum of morphologic subtypes.1,8 Well differentiated ONBs contain circumscribed lobules which are separated by a vascularized fibrous stroma with pseudorosettes and true rosettes.1 Pleomorphism, high mitotic activity and necrosis are infrequently observed, although the latter two features are more prominent in canine and feline ONB as compared with humans.1 Dense core neurosecretory granules and neurite-like cell processes can be observed ultrastructurally.1
Histologic grading (Hyam's classification in humans) is used to gauge prognosis and predict recurrence.1 The Kadish grading scheme (a system used for staging in humans) is based upon the local spread of the neoplasm.5 ONBs of dogs and cats were graded using the Hyam's scheme in one study and evaluated lobular architecture, nuclear pleomorphism, fibrillary matrix, rosettes, mitoses, necrosis and calcification. Grades II and III were the most common in both dogs and cats in a series of 30 cases,1 with feline ONBs showing more differentiation and typically lower grades. Using this grading scheme, the current case would be high grade (III) due to the prominent mitotic activity and necrosis. In that case series, rosettes in feline tumors were more frequently observed than in canine tumors. This case showed frequent rosette formation.
The immunohistochemical findings support the diagnosis of ONB, however, some of the differential diagnoses cannot be entirely ruled out. In the aforementioned case series, feline cases were immunopositive for microtubule-associated protein-2 (MAP-2), Neuronal nuclei antigen (NeuN), and Neuron specific enolase (NSE).1 In our case, neoplastic cells were positive for MAP-2, NSE, Synaptophysin, Cytokeratin, and weakly for NeuN. Neoplastic cells were diffusely negative for Chromogranin. Despite the low specificity of NSE, expression with this marker is used to support the diagnosis of olfactory neuroblastoma.1,5 MAP-2 and NeuN are also considered reliable markers of neuronal and neuroendocrine tumors in humans.1 In one study involving feline esthesioneuroblastoma, NeuN (a nuclear stain) did not stain central regions or cells within rosettes, a pattern which differed from that of dogs.1 Infiltrating populations of neoplastic cells at the periphery of feline ONBs stained with NeuN, however this marker is less useful as a diagnostic marker for this neoplasm in cats.1 The neoplastic cells in this case were diffusely immunopositive for pancytokeratin (AE1/AE2), which is typically a feature of neuroendocrine carcinoma.12 Coexpression of NSE, Neurofilament protein (NF) and cytokeratin in human ONB has been attributed to the possible genesis from olfactory basal cells which are progenitors of olfactory receptor (neuronal) cells and sustentacular (epithelial) cells.8 Rare expression of MAP-2 and NeuN can occur in neuroendocrine carcinomas of humans.1 ONBs are consistently negative for epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA) which were not performed in this case due to lack of availability. Definitive differentiation would require ultrastructural evaluation or immunohistochemistry for EMA or CEA.1,12 Both mitotic count and Ki67 labelling index (a marker of cell proliferation), were found to be much higher in feline and canine tumors as compared with human olfactory neuroblastomas.1 Ki-67 labelling correlated strongly with tumor grade in that study.1,5
FeLV has been associated with olfactory neuroblastoma, however, no causal role was established?.8 In three feline cases of spontaneous olfactory neuroblastoma, numerous mature C type retrovirus particles were identified within the tumors. Two of the three cats were serologically positive for FeLV (the third was not tested).5,8 Olfactory neuroblastoma in humans and animals is similar with regards to morphology, ultrastructure, molecular and immunohistochemical features. Applying human staging and grading schemes may be useful for prognosticating these tumors in veterinary patients. Animal models of ONB may be helpful in the development of new treatment approaches.5
Contributing Institution:
Animal Medical Center
JPC diagnosis:
Nasal cavity and cerebrum: Esthesioneuroblastoma (olfactory neuroblastoma).
JPC comment:
The contributor summarizes olfactory neuroblastoma well and illustrates the difficulty in differentiating esthesioneuroblastoma from neuroendocrine carcinoma. With H&E sections alone, it is not possible to eliminate neuroendocrine carcinoma as a potential differential diagnosis.
With an eye on improving chemotherapeutic targets, a recent retrospective analysis of 23 human olfactory neuroblastomas used DNA sequencing, whole genome RNA microarrays, gene copy number assays, and immunohistochemistry to characterize the molecular characteristics of neoplastic cells. They found that 63% of cases had genetic mutations, including TP53, CTNNB1, EGFR, APC, cKIT, cMET, PDGFRA, CDH1, FH, and SMAD4.11 A different study found a number of olfactory neuroblastoma cells express programmed death-ligand 1 (PD-L1)4, but this was not found in the current study. An independent study determined that 86% of studied olfactory neuroblastomas had deletions in the Dystrophin gene at the DMD locus, which is responsible for the expression of dystrophin.3 The association between this mutation and pathogenesis is not yet clear but may warrant additional research.
Recent investigation into nine canine high-grade olfactory neuroblastomas found variable immunoreactivity of neoplastic cells to chromogranin, NF, synaptophysin, AE1/AE3 cytokeratin, and MAP2. However, all cases that were evaluated for beta-III tubulin (TuJ-1) demonstrated strong cytoplasmic immunoreactivity. Because there are overlapping immunohistochemical profiles of neuroendocrine carcinoma and olfactory neuroblastoma, TuJ-1 may represent a strong candidate for confirming a diagnosis of olfactory neuroblastoma.2
During discussion, the moderator emphasized the importance of standardized reporting for neoplasms. Organized reporting allows for the collection of important information that may help create future grading frameworks and allow the pathologist and clinician to provide important prognostic information to the client. The below example synoptic report was created for this case. Hopefully with standardization and collaboration, sample sizes can be pooled more easily to create statistical analyses with greater power.
|
|
Synoptic Report Example
|
|
Mass size |
2x1x1 cm (nasal) and 1.5x1x1 cm (brain) |
|
How measured |
Grossly |
|
Location |
Nasal cavity/brain |
|
MC |
Up to 115 |
|
Necrosis |
Present |
|
Histologic Grade |
Grade III (human scheme - Hyam's) |
|
Lymphovascular invasion |
Not observed |
|
Extension beyond compartment |
Yes - nasal cavity into brain |
|
How determined |
Imaging, gross histopath |
|
Diagnosis |
Olfactory neuroblastoma |
|
Ancillary tests |
IHC: Synaptophysin, NSE, MAP-2, NeuN+, Chromogranin equivocal, CEA, EMA - |
|
Metastasis |
Not documented with postmortem examination (gross and histopath) |
References:
- Brosinski K et al. Olfactory neuroblastoma in dogs and cats ? a histological and immunohistochemical analysis. J comp Pathol 2012 146:152-159
- Church ME, Veluvolu SM, Durham AC, Woolard KD. Clinical outcomes, ultrastructure and immunohistochemical features of canine high-grade olfactory neuroblastoma. Vet Comp Oncol. 2019;17(4):578-584.
- Gallia GL, Zhang M, Ning Y, et al. Genomic analysis identifies frequent deletions of Dystrophin in olfactory neuroblastoma. Nature Communications. 2018;9:5410.
- London NR, Rooper LM, Bishop JA, et al. Expression of programmed cell death-ligand 1 and associated lymphocyte infiltration in olfactory neuroblastoma. World Neurosurgery. 2020;135:e187-e193.
- Lubojemska A, Borejko M, Czapiewski P, et al. Of mice and men: olfactory neuroblastoma among animals and humans. Vet Comp Oncol. 2016; 14(3):e70-e82. doi:10.1111/vco.12102
- Meuten DJ. Appendix: Diagnostic Schemes and Algorithms. In: Tumors in Domestic Animals, 5th ed. Meuten DJ ed. John Wiley and Sons, Inc, 2017
- Mukaratirwa S, van der Linde-Sipman JS, Gruys E. Feline nasal and paranasal sinus tumours: clinicopathological study, histomorphological description and diagnostic immunohistochemistry of 123 cases. J Feline Med Surg. 2001; 3(4):235-245. doi:10.1053/jfms.2001.0141
- Schrenzel MD, Higgins RJ, Hinrichs SH, et al. Type C retroviral expression in spontaneous feline olfactory neuroblastomas. Acta Neuropathol. 1990; 80(5):547-53.
- Siudak K, Klinger M, Schmidt MJ, et al. Metastasizing esthesioneuroblastoma in a dog. Vet Pathol. 2015; 52(4):692-695.
- Söffler C, Hartmann A, Gorgas D, et al. Magnetic resonance imaging features of esthesioneuroblastoma in three dogs and one cat. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2016; 44(5):333-340. doi:10.15654/TPK-150963
- Topcagic J, Feldman R, Ghazalpour A, Swensen J, Gatalica Z, Vranic S. Comprehensive molecular profiling of advanced/metastatic olfactory neuroblastomas. PLOS One. 2018;13(1): e0191244.
- Wilson D W. Tumors of the Respiratory System. In: Tumors in Domestic Animals, 5th ed. Meuten DJ ed. John Wiley and Sons, Inc, 2017