CASE 4: 16154 (4117663-00)
Signalment:
Seventeen-year-old, male, Chinese-born rhesus macaque (Macaca mulatta) with an unknown breeding history.
History:
This animal was a control animal and blood donor.
Gross Pathology:
N/A
Laboratory results:
N/A
Microscopic description:
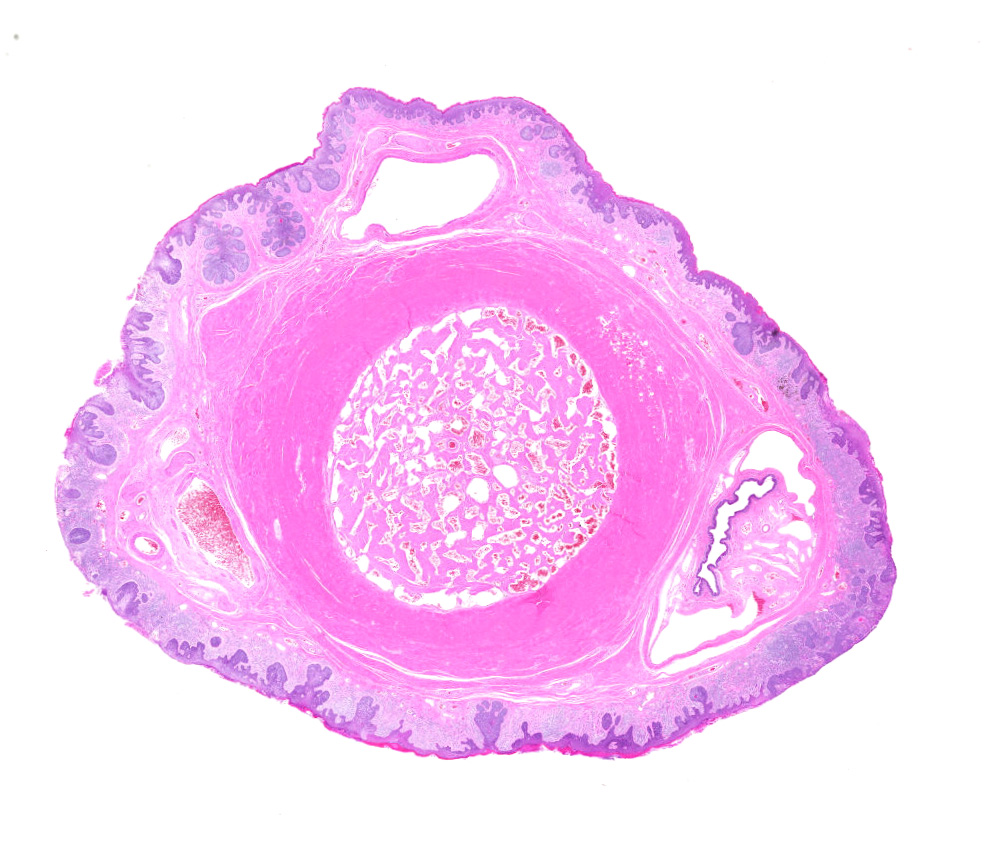
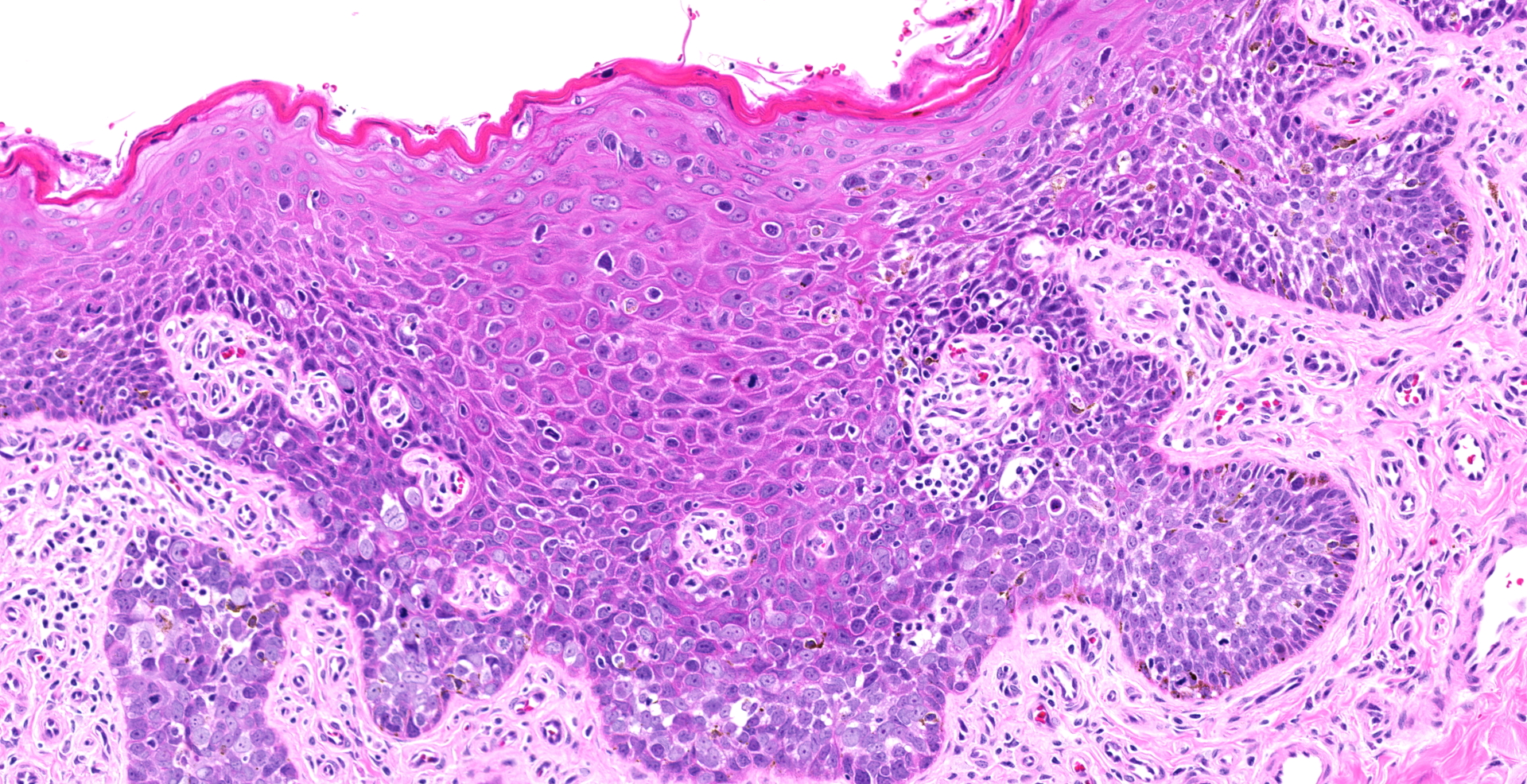
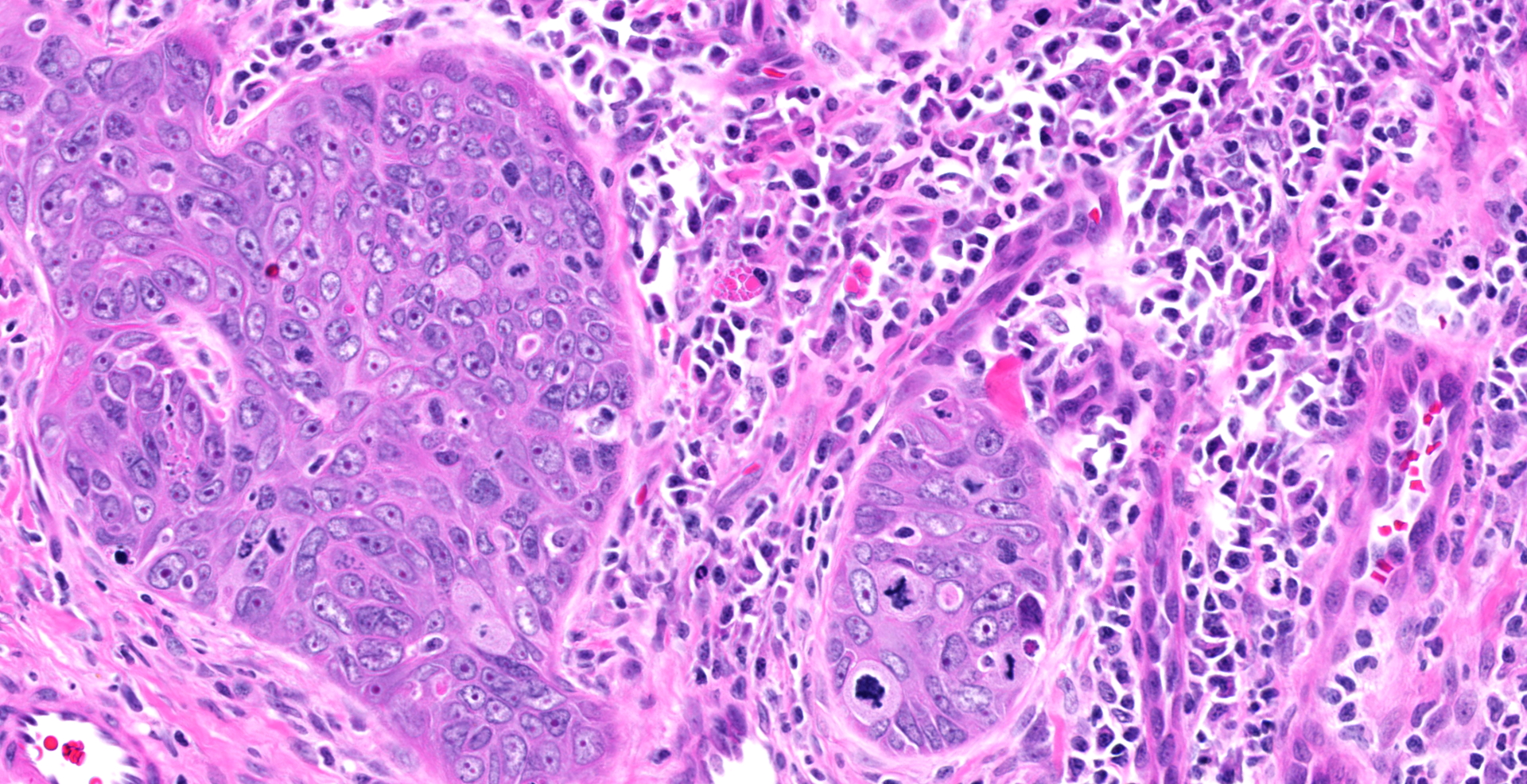
Diffuse hyperplasia of the stratum basale is present in the skin of the penis, in many places forming downward projections which extend into the dermis. In one area, the epithelium is disorganized with loss of polarity and haphazardly arranged, enlarged polygonal cells which vary in size up to 35?m in diameter). These cells have distinct borders, moderate amounts of amphophilic cytoplasm, large vesicular nuclei, and single prominent nucleoli. Mitoses are abundant, at up to 22 per ten 40x fields. Ventral to the urethra, the basement membrane is breached by small cords and clusters of neoplastic epithelial cells. In addition, erosions and ulcers are present, the dermis is infiltrated by lymphocytes, plasma cells and fewer neutrophils, and aggregates of brownish-black pigment are present in the dermis. Clusters of prominent thick-walled capillaries lie beneath an area of ulceration. A keratin pearl is surrounded by neoplastic cells and rare individual cell keratinization (dyskeratosis) is present in the epidermis.
Contributor's morphologic diagnosis:
Epithelial hyperplasia and dysplasia, focal, marked with ulceration, moderate chronic lymphoplasmacytic dermatitis, and focal microinvasive squamous cell carcinoma, penis
Contributor's comment:
Although the histologic findings were suggestive of papillomavirus-induced changes, the PCR from paraffin embedded sections for rhesus papillomavirus type D (RhPV-d) was negative. Immunohistochemical staining of these sections, however, was positive for markers of proliferation (p16 and Ki67) and dysregulation of the tumor suppressor p53. Possible interpretations for the negative PCR result include infection with a non-RhPV-d strain, absence or low levels of virus in sectioned tissues, or the lesions were not of viral etiology.
Papillomaviruses are small, non-enveloped, double-stranded DNA viruses of the family Papillomaviridae which infect epithelial cells. A variety of human papillomaviruses (HPV) are associated with anogenital and oropharyngeal neoplasia.5 Out of the 100+ HPV types, high risk HPVs (HPV 16, 18, 35 and 41) are capable of inducing malignant changes in the human reproductive tract. Penile carcinoma is less well-characterized compared to female reproductive tract carcinomas associated with HPV9, and only thirty percent of penile cancers are associated with HPV.1
The expression of p16 and p53 are associated with HPV-HR (high risk)-induced penile carcinogenesis while p16 negative, p53 positive lesions were identified in HPV-negative cancers.11 In another human study, p16 and high Ki-67 expression were considered efficient diagnostic markers for precancerous lesions in HPV positive individuals.10
In non-human primates, thirteen identified RhPVs belong to the alpha-PV group, and most are unrelated to HPV.3 However, RhPV-1, was found to be closely related to an HPV16 which was isolated from an inguinal lymph node in a rhesus macaque with a penile squamous cell carcinoma.8,12 Cervical intra-epithelial neoplasia in female cynomolgus macaques is associated with the most common type of PV, RhPV-d, which is closely related to RhPV-1 and HPV16 based upon L1 and E6 ORF sequences. RhPV-d is transmissible between macaques, as the viral gene product, E1^E4, was confirmed in two RhPV-d donors and three recipients in cervical cells.15 The initial report of RhPV-1 was from a penile squamous cell carcinoma in a male rhesus macaque,12 but other reports in non-human primates are nonexistent.
Non-HPV premalignant penile lesions in humans are primarily associated with lichen sclerosus which has a predilection for the genital area.7 Penile intraepithelial neoplasia (PeIN) is classified into differentiated PeIN, which are most commonly associated with chronic inflammation, and undifferentiated subtypes (warty, basaloid and mixed warty-basaloid) that are strongly associated with HPV.13 PeINs have also been classified based upon p16/p53/Ki-67 immunohistochemical panels. Differentiated PeIN is typically p16 negative and Ki-67 positive and has variable p53 expression. However, p16 and Ki-67 staining are consistently positive in basaloid and warty PeINs, again with variable p53 positivity.4
Contributing Institution:
http://www.wakehealth.edu/Comparative-Medicine/
JPC diagnosis:
1. Penis: Squamous cell carcinoma.
2. Penis, mucosa: Hyperplasia, circumferential, severe, with dysplasia, and moderate lymphoplasmacytic submucosal inflammation.
JPC comment:
The contributor summarizes this case succinctly. The use of the descriptor microinvasive is used much more commonly in human pathology than in veterinary pathology. While most commonly applied to squamous cell carcinoma in the larynx or the cervix, it has been applied to SCC in other locations as well. The definition varies, but most often includes:
1. The presence of scattered malignant cells just below the basement membrane
2. The presence of malignant cells with no invasion beyond 2mm
3. The presence of malignant cells within 1-2mm of the basement membrane without angioinvasion
4. The presence of discrete foci of malignant epithelium invading through the basement membrane
However, a simpler summarization is an invasive carcinoma extending into the stroma not more than 0.5mm beyond the basement membrane and with no evidence of angioinvasion.14
Papillomaviruses have been associated with penile lesions in other species as well. Young bulls commonly develop fibropapillomas on the head of the penis as a result of infection with bovine papillomavirus 1. While fibropapillomas are benign, Equus caballus papillomavirus 2 is implicated in the malignant transformation to SCC in the penis of the middle-aged horse.6 Similar to this case, p53 is most often overexpressed, and in situ hybridization (ISH) has confirmed E6/E7 viral nucleic acids in neoplastic cells.15
Using Ki67 as a marker of proliferation has been well established in human pathology and is most well characterized in mast cell tumors in veterinary literature. Ki67 is a non-histone nuclear matrix protein expressed in all phases of the cell cycle, except G0. It has a relatively short half-life of 1 hour, and an increased from the baseline proliferative index may indicate neoplasia. In human penis SCC research, a statistically significant threshold of <20% Ki67 immunolabeling correlated with Grade 1 neoplasms, up to 46% for Grade 2, and Grade 3 neoplasms had up to 66% of neoplastic cells immunolabeled. These determinations are useful when attempting to correlate proliferation index with grade and prognosis. While this data may not translate exactly to nonhuman primates, it may serve as a starting point for creating classifications for SCC in nonhuman primates.2
References:
1. Alemany L, Saunier M, Alvarado-Cabrero I, Quiros B, Salmeron J, Shin HR, et al.: Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int J Cancer 2015:136(1):98-107.
2. Berdjis N, Meye A, Nippgen J, et al. Expression of Ki-67 in squamous cell carcinoma of the penis. BJU Int. 2005 Jul;96(1):146-8.
3. Chan SY, Bernard HU, Ratterree M, Birkebak TA, Faras AJ, Ostrow RS: Genomic diversity and evolution of papillomaviruses in rhesus monkeys. J Virol 1997:71(7):4938-4943.
4. Chaux A, Pfannl R, Rodriguez IM, Barreto JE, Velazquez EF, Lezcano C, et al.: Distinctive immunohistochemical profile of penile intraepithelial lesions: a study of 74 cases. Am J Surg Pathol 2011:35(4):553-562.
5. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al.: Global burden of human papillomavirus and related diseases. Vaccine 2012:30 Suppl 5:F12-23.
6. Foster RA. Male Genital System. In: Maxie MG ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, Vol 3. St. Louis, MO: Elsevier. 2016;508-509.
7. Kacerovska D, Michal M, Hora M, Hadravsky L, Kazakov DV: Lichen sclerosus on the penis associated with striking elastic fibers accumulation (nevus elasticus) and differentiated penile intraepithelial neoplasia progressing to invasive squamous cell carcinoma. JAAD Case Rep 2015:1(3):163-165.
8. Kloster BE, Manias DA, Ostrow RS, Shaver MK, McPherson SW, Rangen SR, et al.: Molecular cloning and characterization of the DNA of two papillomaviruses from monkeys. Virology 1988:166(1):30-40.
9. Krustrup D, Jensen HL, van den Brule AJ, Frisch M: Histological characteristics of human papilloma-virus-positive and -negative invasive and in situ squamous cell tumours of the penis. Int J Exp Pathol 2009:90(2):182-189.
10. Lim S, Lee MJ, Cho I, Hong R, Lim SC: Efficacy of p16 and Ki-67 immunostaining in the detection of squamous intraepithelial lesions in a high-risk HPV group. Oncol Lett 2016:11(2):1447-1452.
11. Mannweiler S, Sygulla S, Winter E, Regauer S: Two major pathways of penile carcinogenesis: HPV-induced penile cancers overexpress p16ink4a, HPV-negative cancers associated with dermatoses express p53, but lack p16ink4a overexpression. J Am Acad Dermatol 2013:69(1):73-81.
12. Ostrow RS, LaBresh KV, Faras AJ: Characterization of the complete RhPV 1 genomic sequence and an integration locus from a metastatic tumor. Virology 1991:181(1):424-429.
13. Shabbir M, Minhas S, Muneer A: Diagnosis and management of premalignant penile lesions. Ther Adv Urol 2011:3(3):151-158.
14. Wenig BM. Tumors of the Upper Respiratory Tract, Part B, Larynx, Hypopharynx, and Trachea. In: Diagnostic Histopathology of Tumors, Vol 1, Part 1. Philadelphia, PA: Elsevier. 2013;171.
15. Wood CE, Chen Z, Cline JM, Miller BE, Burk RD: Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. J Virol 2007:81(12):6339-6345.
16. Zhu KW, Affolter VK, GaynorAM, Dela Cruz Jr, FN, Pesavento, PA. Equine Genital Squamous Cell Carcinoma: In Situ Hybridization Identifies a Distinct Subset Containing Equus caballus Papillomaviurus 2. Vet Pathol. 2015;52(6):1067-1072.