Wednesday Slide Conference, Conference 18, Case 4
Signalment:
12-year-old, male, blue fronted Amazon parrot (Amazona aestiva).
History:
Two-week history of not acting right. One week history of acute decline, weight loss, ataxia, increased respiratory effort/rate and loss of deep pain over 24 hours. Radiographs revealed evidence of airsacculitis, and bloodwork showed severe heterophilia. The patient’s condition deteriorated despite antibiotic administration. Euthanasia was elected.
Gross Pathology:
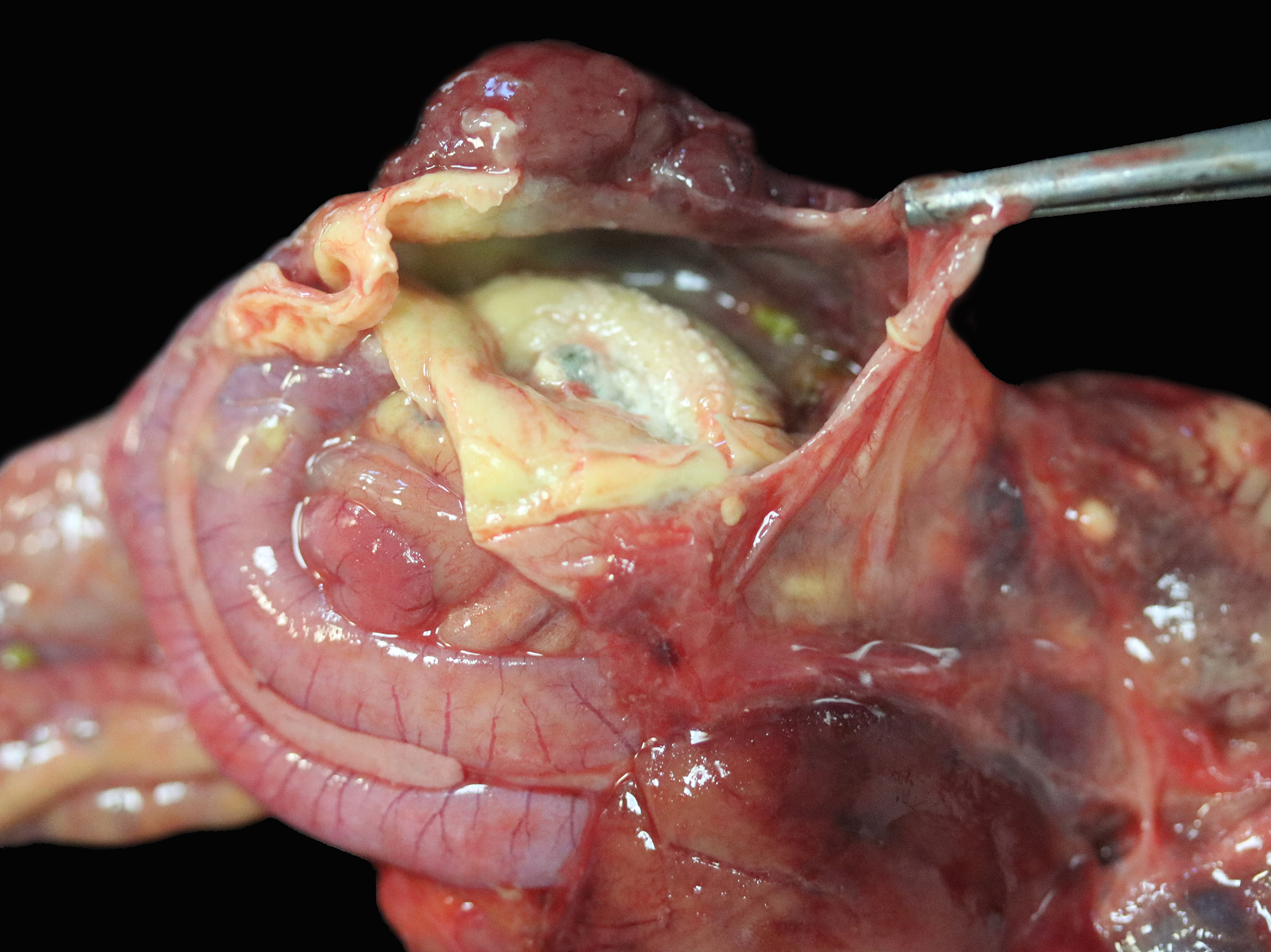
The right and left abdominal air sacs are severely thickened and tan to yellow, with shaggy, irregular projections (airsacculitis). The coelomic cavity contains small amounts (approximately 1 mL) of tan to yellow effusion. Focal regions of yellow to tan thickening are noted over the body wall in the regions of the right and left femur. The right lung is mottled dark red, light red and pale tan. A 0.5 cm diameter, firm, tan nodule is present in the middle section of the lung lobe. Samples from this lobe sink in formalin. The left lung is also mottled light and dark red to brown and has a focal, 0.3 cm diameter tan nodule (presumed granulomatous heterophilic pneumonia). A firm region in the middle section of the lung lobe is observed. Sections from this lobe float in formalin.
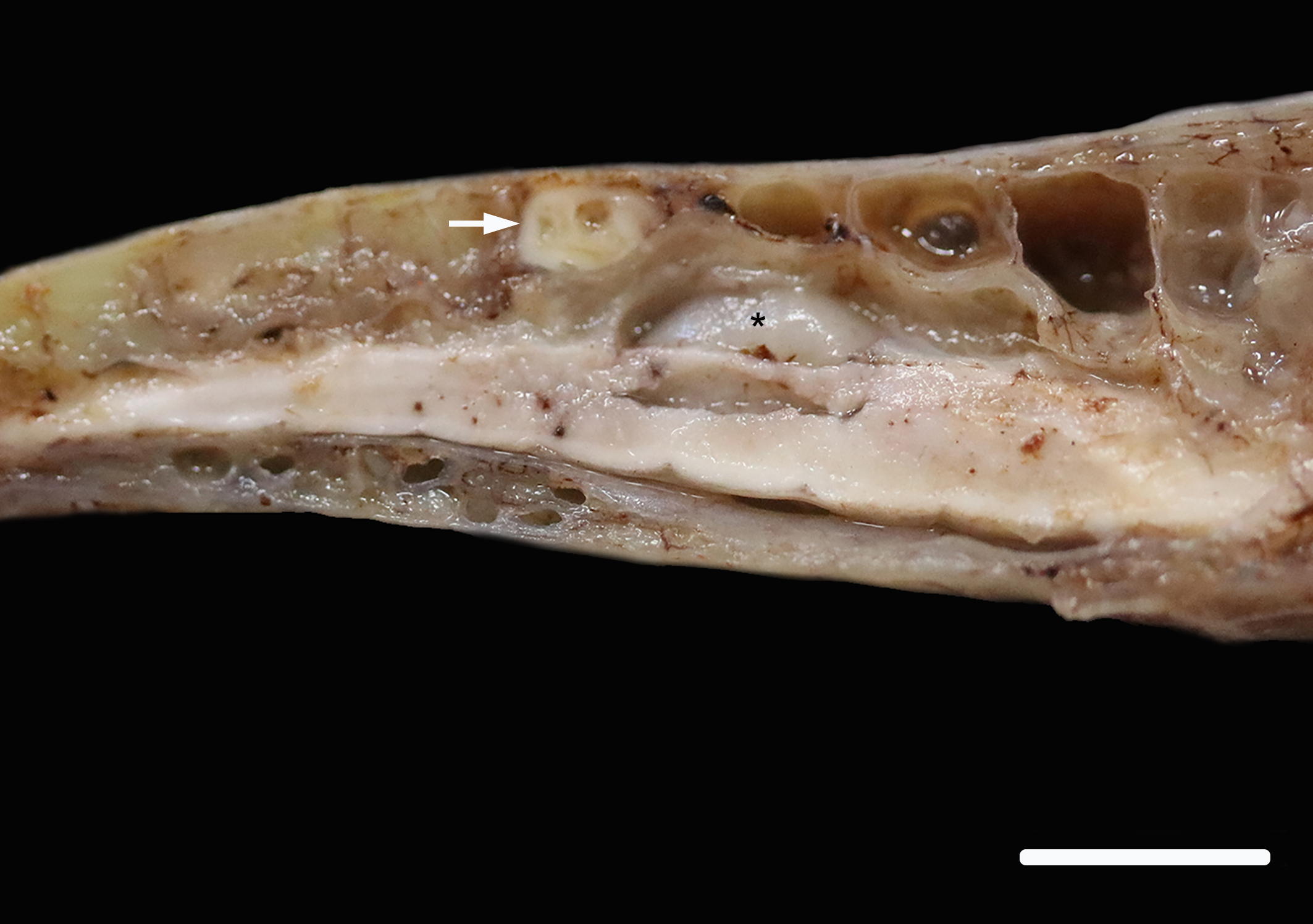
The spinal cord is removed in situ, is fixed, decalcified and sectioned. In the lumbar spine, there is a focal, white, firm nodule in the bone. This nodule overlies and is just caudal to the glycogen body of the spinal cord.
Laboratory Results:
CBC: Hematocrit 38 (range 39-54%), WBC 56.5 K/µL (range 5-15 K/µL), Heterophils 44 K/µL (range 1.5-11 K/µL), Heterophil bands 5.1 K/µL (0 K/µL), Lymphocytes 2.3 K/µL (range 1-10 K/µL), Monocytes 5.1 K/µL (range 0-0.4 K/µL), Eosinophils 0 K/µL (range 0-0.3 K/µL), Basophils 0 K/µL (range 0-0.4 K/µL), thrombocytes adequate, polychromasia slight, no parasites seen.
Microscopic Description:
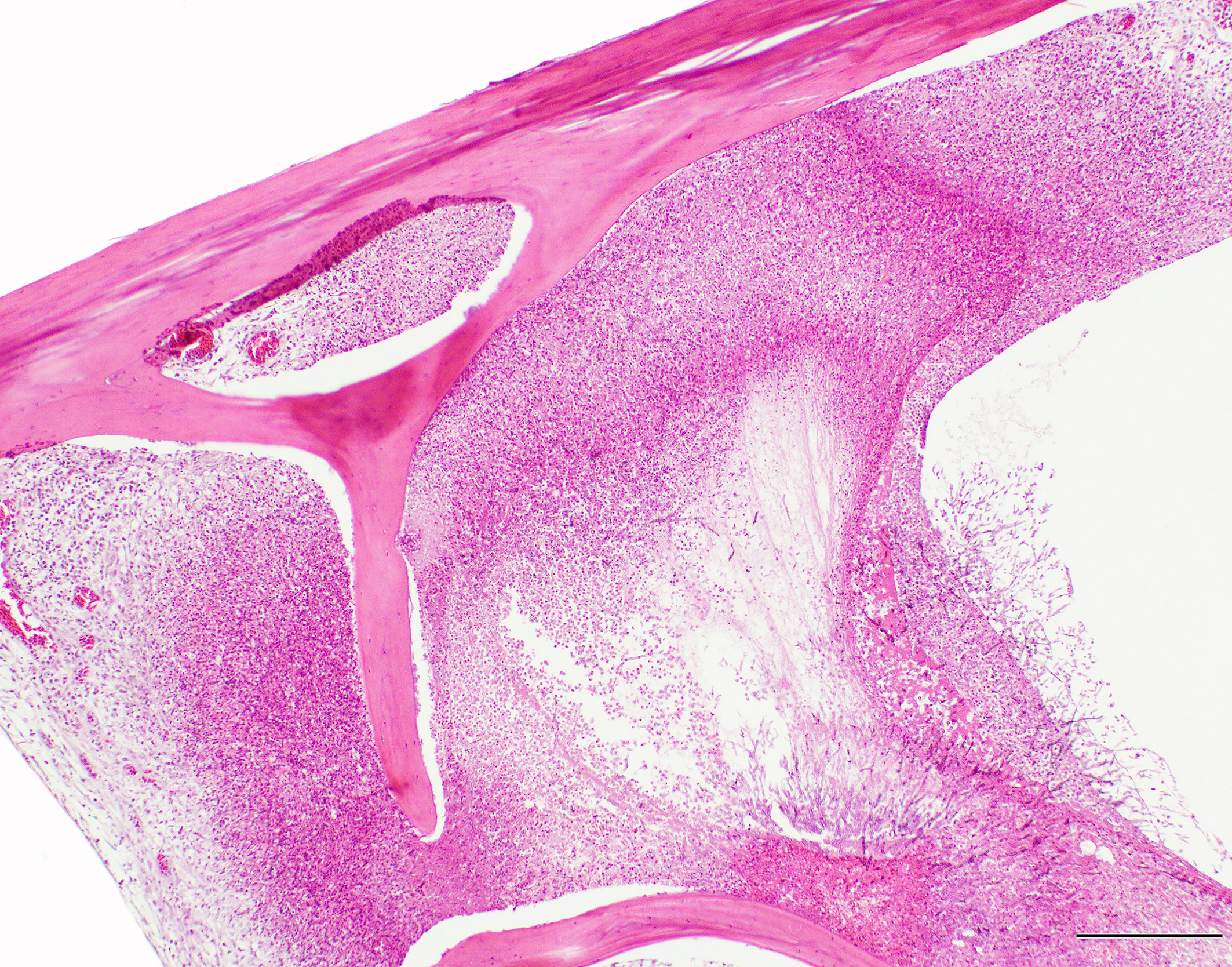
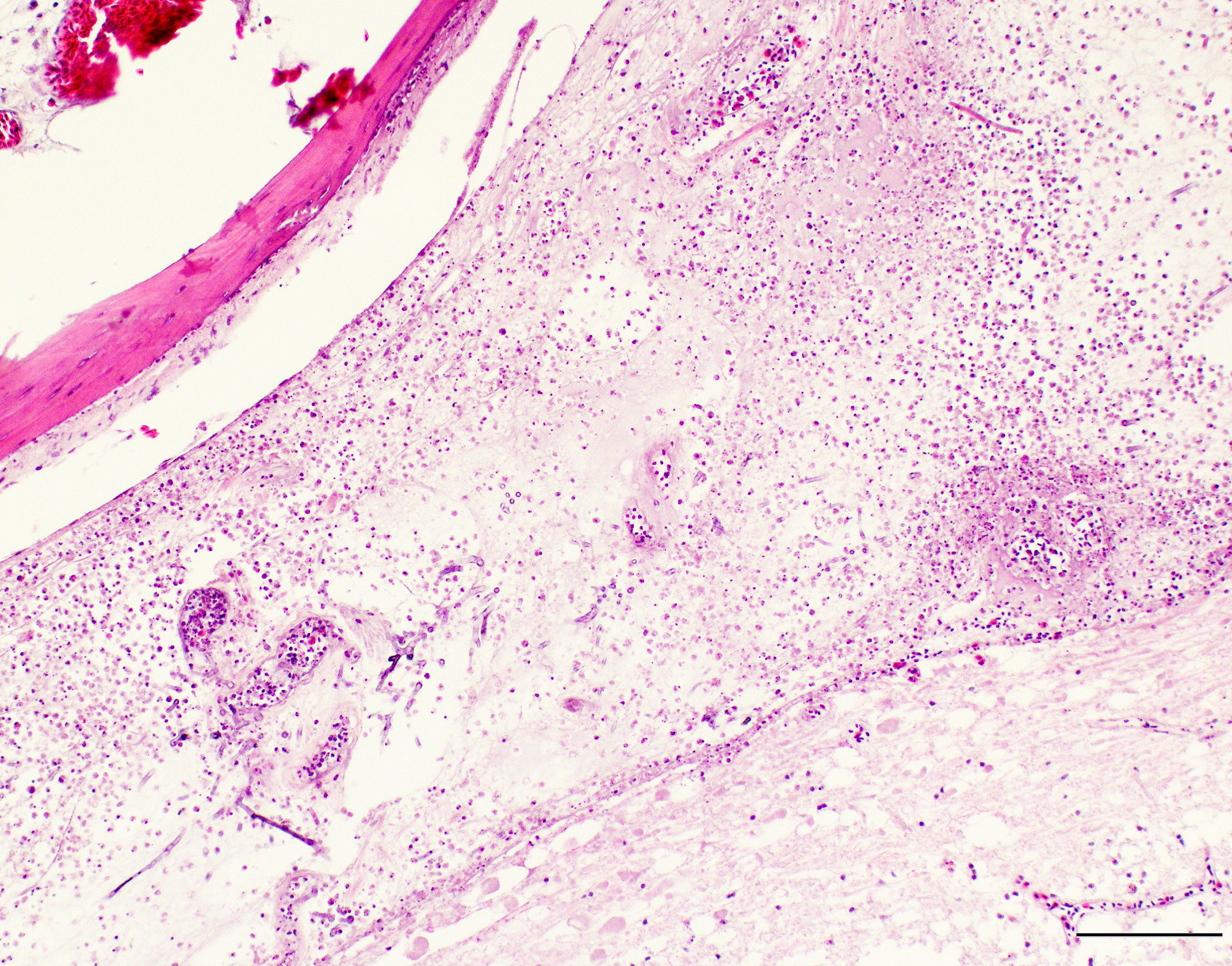
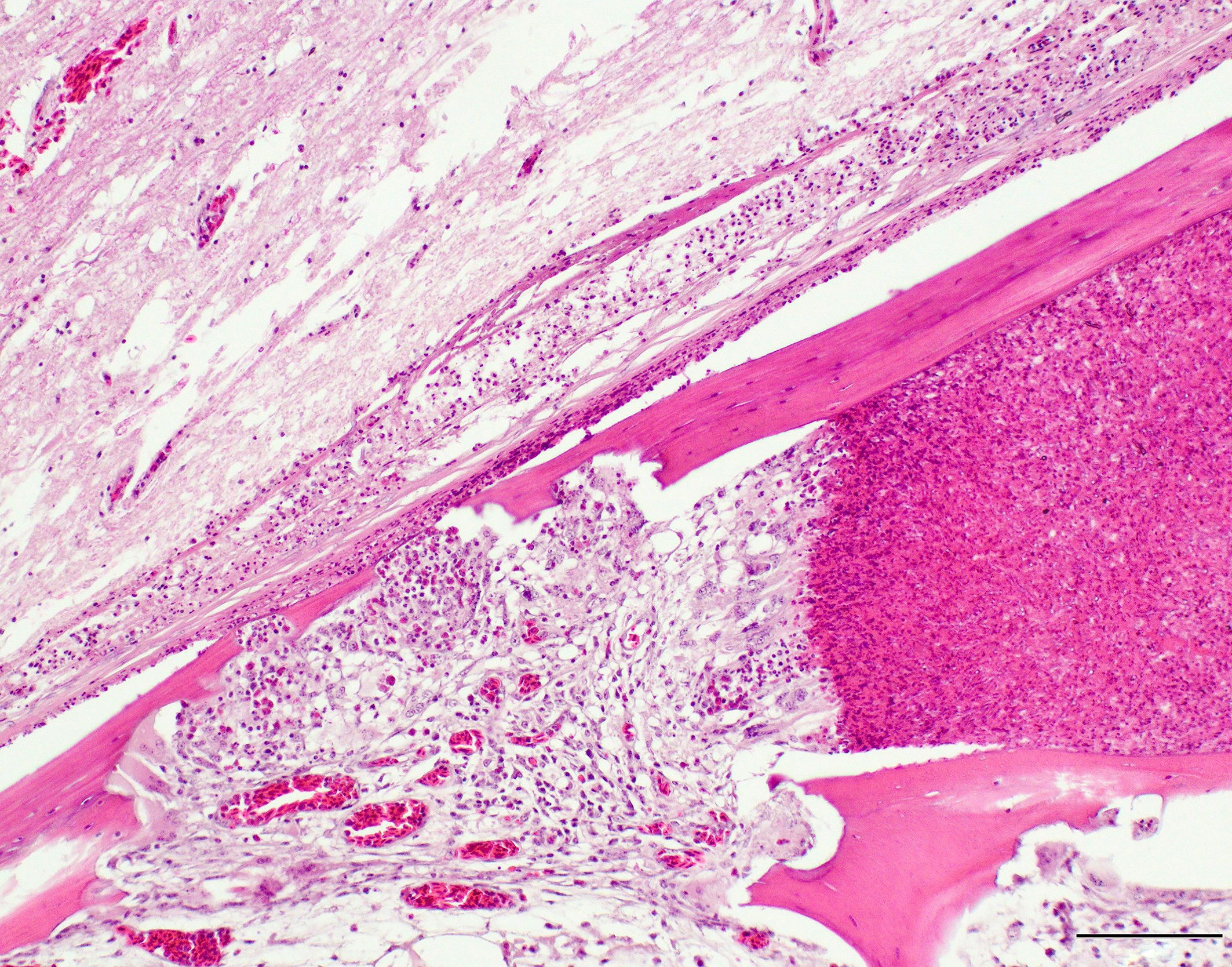
A section of lumbosacral vertebral column and spinal cord are examined. Within the bone of the lumbar vertebral column, a densely cellular region of inflammation is present. The cellular infiltrate comprises predominantly heterophils including degenerate heterophils and fewer macrophages, lymphocytes and plasma cells. Multinucleate giant cells and epithelioid macrophages are occasionally observed, with formation of heterophilic granulomas. Inflammatory populations result in lysis of adjacent bone, with irregular, scalloped margins of attenuated vertebral bone. Additional foci of inflammation are multifocally distributed throughout the vertebral bone within the section. The marrow and pneumatized cavities contain lightly eosinophilic fluid (edema). The inflammation extends to the spinal cord, with heterophils and macrophages infiltrating through the meninges and into the spinal cord parenchyma. In these regions, the spinal cord exhibits myelin vacuolation with regional gliosis including Gitter cells. Axons are enlarged and homogeneously eosinophilic (spheroids). Inflammatory cells are intermixed with fungal hyphae. Hyphae occasionally surround and infiltrate vessel walls, which contain inflammatory populations, hyaline, eosinophilic, acellular material (fibrinoid change) and are surrounded by wispy, loosely arranged, eosinophilic material (edema). Fungal hyphae have parallel walls with infrequent septations, range from approximately 3 to 6 µm in width, and demonstrate acute angle, dichotomous branching, highlighted with a Gomori’s methenamine silver (GMS) stain. Heterophilic, histiocytic inflammation and fungal hyphae are observed at the periphery of and minimally infiltrating into the glycogen body, which also contains small foci of hemorrhage.
Contributor’s Morphologic Diagnosis:
Lumbosacral spine and spinal cord: Osteomyelitis and myelitis, heterophilic,granulomatous, lymphoplasmacytic with myriad fungal hyphae, regional myelin vacuolation with gliosis and spheroid formation, vasculitis with fibrinoid degeneration and edema.
Abdominal airsacs (not included on slide): Airsacculitis, severe, necrotizing, heterophilic, granulomatous with myriad fungal hyphae and conidiophores (morphology consistent with Aspergillus spp.).
Contributor’s Comment:
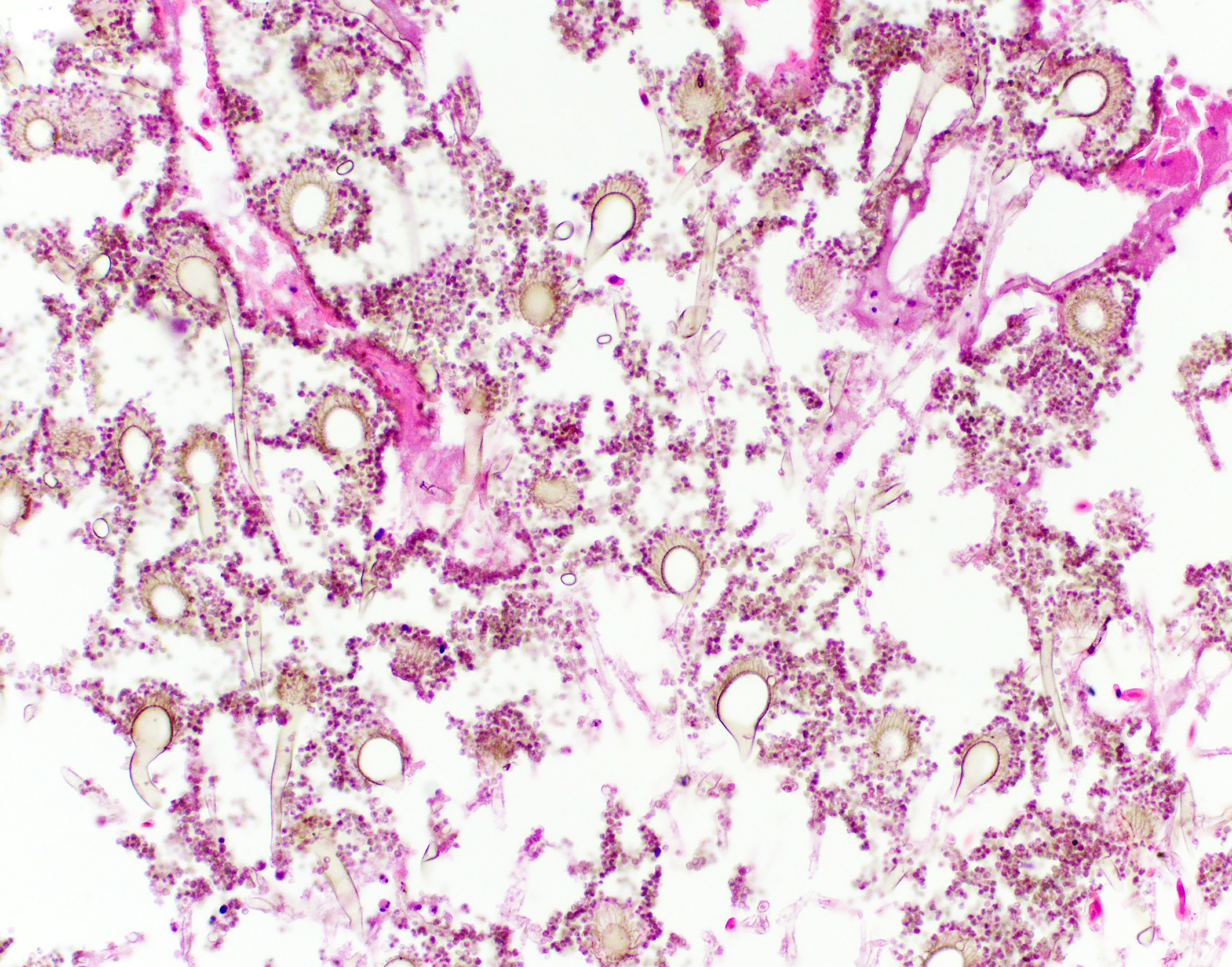
This psittacine bird had a disseminated fungal infection, involving multiple organ systems including the lungs, air sacs, vertebral column, spinal cord, skeletal muscles, coelomic cavity, kidneys, liver, intestines, adrenal glands and thyroid glands. The most severely affected organ system was the respiratory system. Myriad fungal hyphae were present within foci of inflammation. These hyphae contained the morphologic features of Aspergillus species, including narrow width (3-6 µm), parallel walls with septation, dichotomous, acute angle branching and a tendency to be oriented in the same direction.2,4
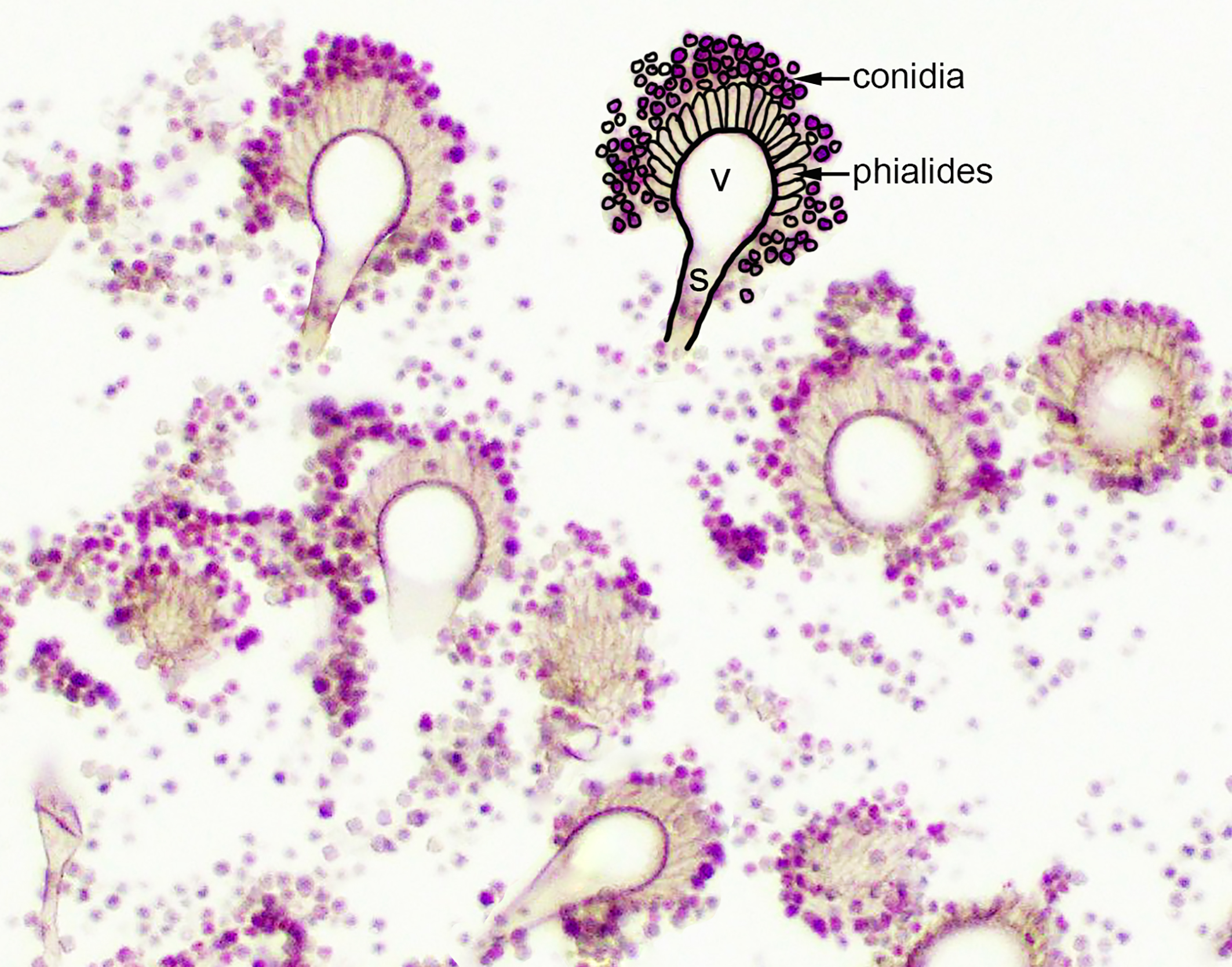
In some locations, conidiophores were observed. Distinctive conidiophores (asexual fruiting bodies) occur on surfaces or cavities exposed to air, such as the air sacs.2,7 Conidiophores consist of a stalk protruding from a mycelial foot cell, with a terminal globose, hemispherical or flask shaped vesicle. Peg-like phialides (formerly sterigmata) arise from the vesicle and may be directly connected and arranged in a single layer (uniseriate) or connected by supporting cells (metulae) and arranged in a double layer (biseriate).2,7 Unbranched chains of conidia are formed from the distal ends of the phialides.2,7 The conidiophores in this case appeared to be uniseriate, which is a feature of the Aspergillus fumigatus group, however, definitive diagnosis requires ancillary testing, as there is overlap in histomorphologic features of Aspergillus conidiophore morphology (see below).
A sequel to the fungal infection in this case was osteomyelitis of the lumbosacral spine with extension to the spinal cord. Frequent sites of secondary involvement in birds include the coelomic cavity, central nervous system (CNS), liver, intestines, kidney, pneumonic bone, adrenal glands and vertebral column.8 In the submitted case, osteomyelitis was most severe in the lumbosacral portion of the spine (in the region of the corpus gelatinosum or glycogen body), but inflammation was also found in the thoracic and cervical segments. Inflammation of the lumbar vertebral column led to osteomyelitis and regional myelitis, with involvement of the glycogen body. Inflammation of the glycogen body is of uncertain significance in the context of the clinical signs. The glycogen body (corpus gelatinosum) is an ovoid, circumventricular, gelatinous structure of unknown function embedded in the lumbosacral region of the spinal cord, occupying the rhomboid sinus.5 The corpus gelatinosum is dorsal to the spinal cord and the ventral portion encloses the central canal. Peripheral and central cellular zones have been described. Cells of this structure contain abundant, pale, vacuolated cytoplasm and peripheralized nuclei. These glycogen containing cells are hypothesized to arise from specialized, modified glial cells (astrocytes).5 Although the function is unknown, hypotheses include an energy source for the central nervous system (CNS), transmission of hydrostatic pressure changes during movement, roles in neuron metabolism, and myelin formation.5 Extension of the fungal infection from the abdominal airsacs to the skeletal muscle of the proximal limbs also likely contributed to the ataxia noted in the history.
Aspergillosis is a non-contagious fungal disease that affects captive and wild birds.1,3 The fungus is ubiquitous, opportunistic and saprophytic, and exposure to large numbers of fungal spores can result in infection. In birds, aspergillosis is most commonly caused by Aspergillus fumigatus, and less commonly Aspergillus niger, Aspergillus flavus, Aspergillus nidulans, Aspergillus glaucus, Aspergillus oryzae and others.1,3,7–9 A recent report detailed a case of Aspergillus pseudoviridinutans (a cryptic species of Aspergillus within section Fumigati) causing rhinitis in an orange winged Amazon parrot.6 Some avian species are thought to be predisposed including Amazona sp., Psittacus erithacus, Pionus sp., as well as species that originate from arid environments.6,9 Other predisposing factors include increased concentrations of environmental spores, a warm environment, improper humidity, poor ventilation, poor sanitation, and long-term storage of feed. Impaired immunity can also predispose to development of Aspergillosis, and has been reported with steroid administration, metabolic bone disease, inadequate diet, overcrowding, shipping, starvation, thermal discomfort, infections, toxins and trauma, among others.1,3,6,8,9
The main route of infection is thought to be inhalation. As the spores of Aspergillus fumigatus are smaller than spores of other Aspergillus spp, some may reach the lungs and air sacs.1,6 Similar to this case, the air sacs are a common site of primary infection (inhaled air reaches caudal thoracic and abdominal air sacs before the pulmonary epithelial surfaces).1 Leukocytosis is a common clinical feature, and is often severe (20,000 to more than 100,000 white blood cells per microliter) with a left shift, monocytosis and lymphopenia. Leukocytosis in this case was 56.5 K/µL, with heterophilia, a left shift, and monocytosis. Serological tests for aspergillus have been developed, however, false negative results can occur.1 Serum protein electrophoresis may reflect an acute phase response, often observed with aspergillosis, which includes increased globulins with reduced albumin, increases in alpha 2 globulins and increased serum amyloid A levels.3
Gross lesions are variable, but generally comprise granulomas of the lungs and air sacs. In acute infections, miliary granulomas may be distributed throughout the respiratory tract.1,8,9 White to yellow plaques (scutulae) may be seen within the respiratory tract or over serosal surfaces. Air sacs are often thickened and may contain caseous exudate or visible mycelial formation.8,9 Nodules within the lungs may have caseous, consolidated or necrotic centers. Histologically, large numbers of degenerate heterophils are typically present, accompanied by necrosis, multinucleated giant cells, epithelioid macrophages, and variable numbers of fungal organisms.8,9 In aerated organs, aggregates of radiating hyphae (the sunburst pattern) frequently occur.1,2 If the infection is not eliminated in the respiratory tract, disseminated infection can occur, both by direct extension through the air sac wall and hematogenous spread.1 More information is needed regarding virulence factors of avian aspergillosis. Conidia of Aspergillus fumigatus may be resistant to respiratory macrophage-induced killing.1 Invasion of blood vessels is a frequently reported sequel,1,2 and was observed in this case.
Deposition of calcium oxalate crystals in association with Aspergillus spp has been documented in domestic animals and humans.2,7 Aspergillus spp synthesize oxalic acid as a product of the tricarboxylic acid cycle, resulting in precipitation of calcium oxalate crystals, which may cause tissue damage.7 The crystals are needle-like and may have wheat-sheaf or rosette arrangements, are clear to pale yellow and strongly birefringent under polarized light.2,7 In one study, cases of avian aspergillosis with oxalate crystal formation occurred commonly in respiratory tissues that interfaced with air (nasal sinus, trachea, syrinx, lung, air sac).7 Sparse or colorless crystals can be difficult to identify without routine polarization. In the aforementioned study, calcium oxalate crystals were found in 11 of 16 avian cases after retrospective review, however, few were identified on the initial report.7 Thus, routine polarization of slides in cases with Aspergillus infections in respiratory tissues is recommended.7
Definitive diagnosis of aspergillosis in birds is based on fungal identification and documentation of fungal organisms within the associated lesions. Hyphal morphology of Aspergillus is similar to that of other fungi in the hyalohyphomycosis group. Some studies found that hyphae morphologically consistent with Aspergillus spp were identified as other genera with culture or PCR.4 The presence of conidiophores allows definitive diagnosis of the genus Aspergillus, however, there is substantial overlap in the morphologic features of the conidiophores of different Aspergillus species, including seriation, phialides arrangement and pigmentation.7 Thus, conidia morphology cannot be relied upon for speciation of Aspergillus.2,7 Fungal culture from clinical samples is considered to be the gold standard for fungal identification, however, the time frame for these cultures is often long. Additional options include use of molecular assays, immunohistochemistry and in-situ hybridization from formalin fixed, paraffin-embedded (FFPE) tissues and cytology slides. PCR using primers that target and amplify conserved portions of the fungal genome (internal transcribed spacers and the 5.8S rRNA gene) followed by sequencing is another potential method of identification.3,4,6 High quality fungal DNA was able to be obtained from FFPE tissues in 70% of cases in one study, which correlated with the histological identification in 62% of cases. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) is an additional potentially useful tool, however, is currently only available for some Aspergillus fumigatus related species.6 Given the presence of conidiophores, additional ancillary tests for speciation were not requested in this case.
Contributing Institution:
www.amcny.org
JPC Diagnosis:
Synsacrum, lumbosacral spinal cord: Osteitis and meningomyelitis, heterophilic, necrotizing, acute, multifocal, severe with vasculitis and fungal hyphae
JPC Comment:
The contributor provides an excellent summary of the case along with many helpful figures. The framing of the entire synsacrum and glycogen body on the slide that mirrors the gross image is simply a bonus for us.
We approached our morphologic diagnosis slightly differently and emphasized (meningo)myelitis and osteitis separately. In contrast to Case 2, the bone in this section is pneumatic and lacks marrow elements. As osteomyelitis best describes inflammation of the osseus medulla (including the marrow), we made this distinction. In our review of the slides we did not observe oxalate crystals, though this may reflect a lack of free calcium to utilize in the TCA cycle (e.g. from adjacent hemorrhage and cell loss).
We covered Aspergillus in the guttural pouch of a horse this year in Conference 1, Case 3. Both cases feature vascular lesions, though the degree of infarction and secondary changes are much more pronounced in that horse than in this parrot. This likely reflects the route of entry via extension of the air sac versus direct hematogenous entry.
Conference participants should have been able to recognize this histologic section as “pelvis” or “synsacrum” based on either of two histologic features: the presence of the glycogen body, which is located in the lumbosacral spinal cord, or the fusion of the vertebral bodies, which represents the synsacrum.
References:
- Beernaert LA, Pasmans F, Waeyenberghe LV, Haesebrouck F, Martel A. Aspergillus infections in birds: a review. Avian Pathol. 2010;39(5):325–331.
- Chandler FW, Kaplan W, Ajello L. A colour atlas and textbook of the histopathology of mycotic diseases. Wolfe Medical Publications Ltd.
- Hauck R, Cray C, França M. Spotlight on avian pathology: aspergillosis. Avian Pathol. 2020;49(2):115–118.
- Hoffmann AR, Ramos MG, Walker RT, Stranahan LW. Hyphae, pseudohyphae, yeasts, spherules, spores, and more: A review on the morphology and pathology of fungal and oomycete infections in the skin of domestic animals. Vet Pathol. 2023;60(6):812–828.
- L E. Structural Insights of the Glycogen Body in Domestic Chicken. J Cytol Histol. 2016;2016(01).
- Langlois I, Barrs VR, Dufresne PJ. Rhinitis due to Aspergillus pseudoviridinutans in an orange-winged Amazon parrot (Amazona amazonica). Me?d Mycol Case Rep. 2020;30:46–50.
- Payne CL, Dark MJ, Conway JA, Farina LL. A retrospective study of the prevalence of calcium oxalate crystals in veterinary Aspergillus cases. J Vet Diagn Investig. 2017;29(1):51–58.
- Reavill DR, Dorrestein G. Psittacines, coliiformes, musophagiformes, cuculiformes. In: Pathology of wildlife and zoo animals. Elsevier 775–798.
- Schmidt RE, Struthers JD, Phalen DN. Pathology of pet and aviary birds. John Wiley & Sons.