Signalment:
Gross Description:
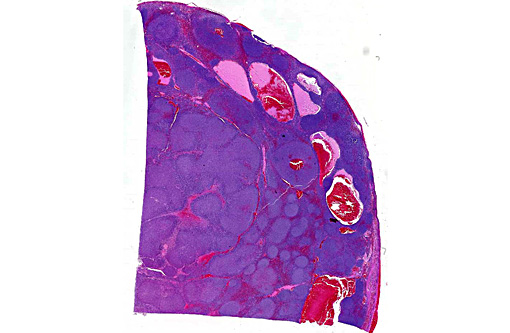
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Indolent lymphomas in dogs include follicular, mantle cell, marginal zone, and T-zone lymphomas. Valli et al examined 66 dogs with indolent lymphoma which included 33 dogs with nodal MZL and 13 cases of splenic MZL; however, only 3 of these cases had outcome data available.(9) Two recent studies describe SMZL in 5 and 34 dogs, respectively, and provide better insight into clinical characteristics and outcome of this disease.(4,6) In the larger study by OBrien et al, the overall median survival time (MST) after splenectomy was 383 days and dogs that had MZL as an incidental finding had a longer MST (1,153 days) compared to dogs with clinical signs associated with MZL (309 days). Other factors such as lymph node involvement, hemoabdomen, adjuvant chemotherapy, and concurrent malignancies did not influence survival.(4)
Assessment of tissue architecture is needed for a diagnosis of MZL, and therefore, histopathology is required. MZL has a distinct nodular pattern in which the lighter-staining neoplastic marginal zone cells form a dense cuff around small foci of darkly stained mantle cells (fading follicles). The neoplastic marginal zone lymphocytes are intermediate in size, with nuclei measuring approximately 1.5 times the diameter of a red blood cell, and have a single prominent central nucleolus. Benign marginal zone hyperplasia (MZH) has a similar architectural appearance, although the expanded marginal zone is heterogeneous, containing a mixture of small and intermediate sized lymphocytes.(10) Differentiating between MZH and MZL is challenging because MZL arises on the background of MZH. Therefore, immunophenotyping and molecular clonality are ultimately required for a definitive diagnosis.
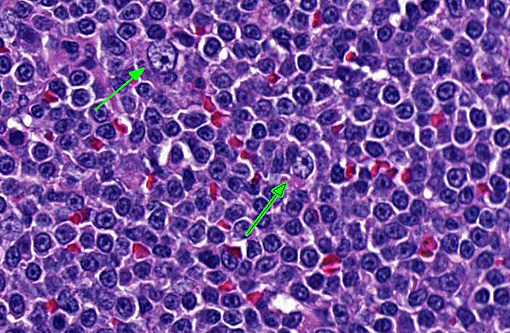
This case highlights the difficulty in differentiating between MZL and MZH and this distinction is especially difficult in the spleen of dogs. Lymphoid nodular hyperplasia and complex nodular hyperplasia (-�-�fibrohistiocytic nodules) are common in the canine spleen; it is possible that many cases of nodular hyperplasia contain areas of MZL. In this case, the architectural feature of homogenous coalescing marginal zone cells was more suggestive of MZL than MZH. The fields in which there were up to 2 mitotic figures further supported this diagnosis, and is likely indicative of later stages of disease development, as mitoses increase with disease progression.(9,10)
As expected, the cells were CD79a positive and CD3 negative, indicating a B cell phenotype. Tissues were sent to the Leukocyte Antigen Biology Laboratory at UC Davis. Molecular clonality analysis of IgH2, IgH3, and KDE (B cell) revealed polyclonal rearrangements, which suggested a reactive process rather than a neoplasm. However, upon review of the histopathological and immunohistochemical findings, the reviewing pathologists at UC Davis were also highly suspicious of MZL and that the PARR testing might be a false negative result. The OBrien paper also found a subset of cases (27%) that did not demonstrate a clonal population (pseudoclonal and polyclonal rearrangements)(3), which is consistent with the published sensitivity of this PCR-based test.(2,8) In that study, the polyclonal rearrangements were attributed to a mutation in V or J segments of Ig. Similarly, the most likely reasons for false negative results in this case are mutation of gene segments that are not covered by the primer sets or mutation of primer sites during somatic hypermutation. In support of this suspicion is the fact that the IgH3 locus did not show a robust polyclonal curve as would be expected in a hyperplastic lesion, but instead had non-reproducible peaks of variable height within a weak polyclonal background.
JPC Diagnosis:
Conference Comment:
Using the grading criteria based on mitotic figures per single 400x field (indolent = 0-1, low = 2-5, intermediate = 5-10, high >10) and cell size determined by comparison to red blood cells (small = 1xRBC, intermediate = 1.5xRBC, large ⥠2xRBC), we identified this neoplasm as intermediate size and low grade, with definitive classification of MZL requiring immunophenotyping and molecular clonality testing.(8)
References:
1. Bennetta M, Schechterb GP. Treatment of splenic marginal zone lymphoma: splenectomy versus rituximab. Semin Hematol. 2010;47:143147.Â
2. Burnett RC, Vernau W, Modiano JF, Olver CS, Moore PF, Avery AC. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol. 2003;40:32-41.
3. Moore AS, Frimberger AE, Sullivan N, Moore PF. Histologic and immunohistochemical review of splenic fibrohistiocytic nodules in dogs. J Vet Intern Med. 2012;26:1164-1168.Â
4. OBrien D, Moore PF, Vernau W, Peauroi JR, Rebhun RB, Rodriguez Jr. CO, Skorupski KA. Clinical characteristics and outcome in dogs with splenic marginal zone lymphoma. J Vet Intern Med. 2013;27:949954.
5. Osciera D, Owenb R, Johnson S. Splenic marginal zone lymphoma. Blood Reviews. 2005;19:3951.
6. Stefanello D, Valenti P, Zini E, Comazzi S, Gelain ME, Roccabianca P, et al. Splenic marginal zone lymphoma in 5 dogs (2001 2008). J Vet Intern Med. 2011;25:9093.
7. Traverse-Glehena A, Baseggiob L, Sallesc G, Felmanb P, Bergera F. Splenic marginal zone B-cell lymphoma: a distinct clinicopathological and molecular entity: recent advances in ontogeny and classification. Current Opinion in Oncology. 2011;23:441448.
8. Valli VE, Kass PH, Myint MS, Scott F. Canine lymphomas: Association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet Pathol. 2013;50(5):738-748.
9. Valli VE, Vernau W, de Lorimier LP, Graham PS, Moore PF. Canine indolent nodular lymphoma. Vet Pathol. 2006;43:241256.
10. Valli VE, Veterinary Comparative Hematopathology. 1st ed. Ames, IA: Blackwell Pub; 2007.