Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2018-2019
Conference 22
5 April, 2019
CASE I: 13-018-6 32 (JPC 4113194 ).
Signalment: Adult male, Rattus norvegicus, Norway rat
History: This animal was trapped and euthanized as part of the pest control program on the outskirts of the Veterinary Medical facility of the Armed Forces Research Institute of Medical Sciences (AFRIMS) in Bangkok, Thailand. The animal appeared well muscled and healthy. Necropsy findings are representative of the over 100 Norway and Black rats that have been captured, euthanized and evaluated since 2012.
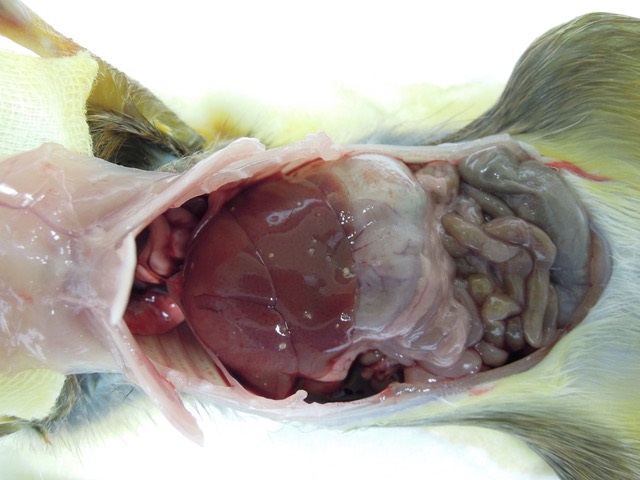
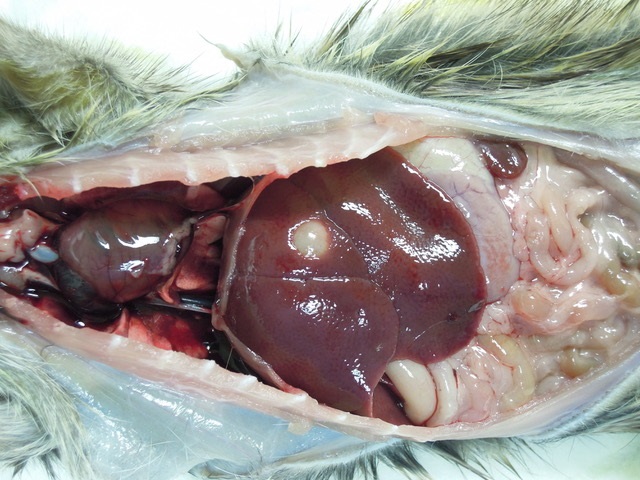
Gross Pathology: Macroscopic findings in this animal consisted of small white cysts (3-5 mm) protruding from the hepatic parenchyma. In this particular case, the cysts were not incised so as to maintain the architecture of the parasitic cyst microscopically, however in other animals incision of the cyst allowed for extrusion of an individual larval cestode consistent with Cysticercus fasciolaris. Additional findings in this animal consisted of both adult nematodes and cestodes within the lumen of the intestinal tract. Blood smears revealed the presence of flagellates etiologically consistent with Trypanosoma lewisi and fecal examination of the animal yielded myriad Strongyle spp. and Strongyloides spp. eggs. No other significant findings were observed.
Laboratory results: NA
Microscopic Description:
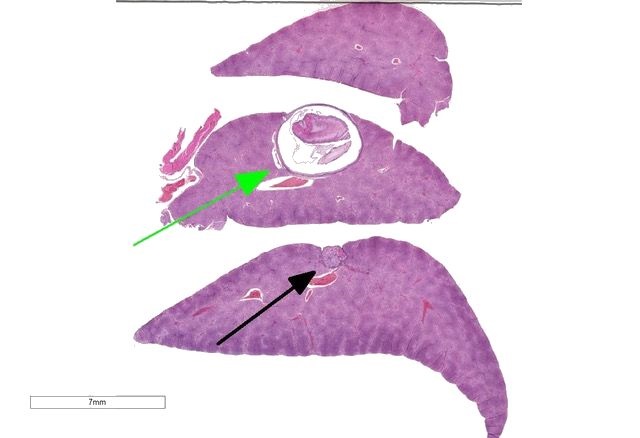
The slide consists of three sections of liver.
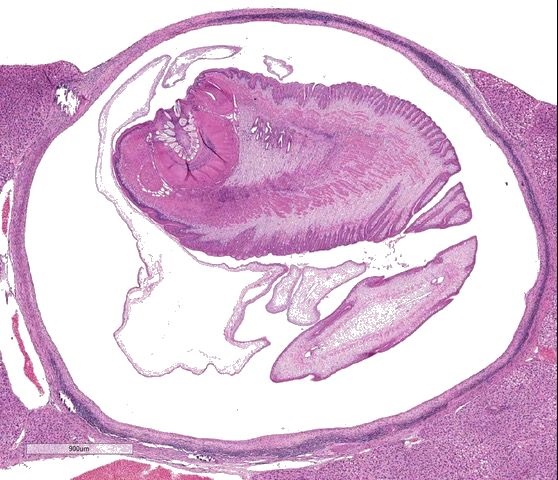
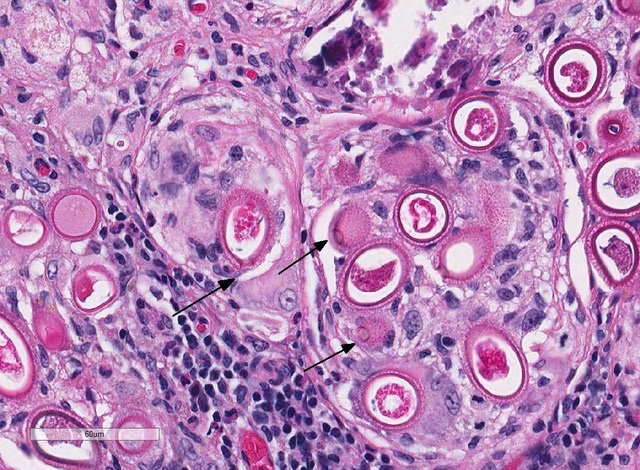
Liver: Focally, markedly expanding and extruding the hepatic capsule, replacing and compressing 20% of the parenchyma is a 3 mm, thin-walled parasitic cyst that contains several cross sections of a larval cestode including its anterior end (cysticercus). The cestode is characterized by measuring approximately 1.5 mm in diameter, lacking a pseudo coelom or a digestive tract. The parasite strobilium is bounded by an undulated tegument which contains the spongy parenchyma. There are streams of muscle fibers which separate the medullary from the cortical regions (typical of Cyclophyllidium), both of which are interspersed with frequent 10 µm, ovoid structures containing central basophilic amorphous material surrounded by a clear halo (calcareous corpuscles). The section also contains a rostellum with 25 armed hooks evident. To one side of the rostellum is a muscular sucker. The cyst wall is characterized by being approximately 150-200 µm wide composed of dense fibroblasts, an internal, circumferential, dense aggregate of lymphocytes, frequent scattered plasma cells, macrophages, fewer eosinophils and occasional hemosiderophages. Multifocally, the cyst wall is disrupted by fractured foci of deeply basophilic mineral surrounded by the previously described inflammatory cells. Exterior to the cyst wall, there are frequent small caliber vessels and lymphatics which are ectatic. The extant hepatic parenchyma is affected by mild centrilobular hepatocellular lipidosis, diffuse glycogenosis and scattered sinusoidal hemosiderophages.
Within the subcapsular region of another section on the slide is a focal lesion consisting of coalescing granulomas characterized by a central core of eosinophilic cellular debris (necrosis), deeply basophilic fragmented spicular material (mineral) or frequent 30 x 80 µm bipolar operculated, thick-shelled, morulated nematode eggs. The central material is bounded by large epithelioid macrophages, few multinucleated giant cells, streams of reactive fibroblasts, and dense aggregates of lymphocytes, plasma cells, hemosiderophages, few eosinophils and rare mott cells. Multifocally, the fibrosis extends into the surrounding parenchyma, dissecting hepatic lobules, individualizing scattered hepatocytes, expanding rare portal areas and is admixed with focally extensive biliary hyperplasia, and small numbers of the previously described inflammatory cells.
Contributor’s Morphologic Diagnosis:
Liver: Cysticercus, focally extensive, etiology consistent with Cysticercus fasciolaris, Rattus norvegicus, rodent
Liver: Granulomas, coalescing, focally extensive, subcapsular, with intralesional bioperculated nematode eggs etiology consistent with Capillaria hepatica
Contributor’s Comment: Preliminary findings in an ongoing histopathological survey of trapped rats in a single point in central Bangkok have found a wide range of helminthic disease, several of which have zoonotic potential. Multiple historical studies have been conducted in South East Asia looking at prevalence of disease in rodents evaluated however there are few that have concentrated on Thailand and Bangkok in particular.2,8,16 It is difficult to assess the overall rat population in this megacity in which there are estimated to be around 10 million human inhabitants, however the rats are an ever present feature of the city, especially during the wet season. The literature describes urban rat populations as being culpable for the spread of a range of zoonoses, to include Leptospira interrogans,7,8 Yersina pestis,7,8 Rickettsia typhi,7,8 Bartonella spp.,7,14 Streptobacillus moniliformis,7,12 Trypanosoma lewisi,8,9,13,16 Angiostrongylus cantonensis,7,16,21 Capillaria sp.1,3,7,15,16,18,19,20 and Taenia sp.3,14,16,19,21 as well as other cestodes such as Hymenolepis spp.2,3,7,21 and Rodentolepis spp.2,7 This is purely a partial list to illustrate the enormity of the potential collision between vector and victim in a concentrated urban environment such as Bangkok. Humans are susceptible to these pathogens in a variety of ways depending on the pathogenesis of the organism in question, however with regards to the helminths, consumption of food or drinks contaminated with rodent droppings or urine is the primary route, with a potential for infection through the eating of bushmeat. In parts of Thailand, this practice includes consumption of rat meat.
The liver of this particular animal does not present a diagnostic challenge, however, it does provide the anatomic pathologist and certainly the veterinary pathology resident with an interesting description of a parasitic coinfection.
The metacestode stage of Taenia taeniaformis is termed Cysticercus fasciolaris but also has been referred to as Taenia crassicollis, Hydatigera taeniaformis and Strobilocercus fasciolaris. Infection with the larval form of Taenia solium, T. saginata, T. crassiceps, T. ovis, T. taeniaeformis or T. hydatigena is termed as cysticercosis. The larvae stages of these cestodes are called cysticerci which are fluid-filled vesicles containing a single inverted protoscolex. Apart from cases of infection of the eye, the ventricles or the subarachnoid space of the brain, these cysts are bounded by a fibrous capsule and rarely an inflammatory response while the parasites are alive. Once they die, there can be a combination of chronic inflammation and mineralization of the lesions.
The definitive hosts of the parasite are members of the felid family to include domestic cats and lynx, but foxes, stoats and other carnivores have been recognized as proficient in transmitting the parasite. In this particular case in which an urban cycle is occurring, the domestic cat is the likely definitive host although the stray dog population in Bangkok is significant. A range of small mammals can act as intermediate hosts in South East Asia, to include various species of murid and cricetid rodents to include rats, and mice as well as insectivores such as bandicoots and lagomorphs.
There is a single instance in the literature of human infection with the larval form of T. taeniaformis and as such the parasite is not felt to represent a significant zoonotic risk, although there are several reports of humans having adult parasites in their intestinal tract.22
The second etiology noted in these sections are Capillaria hepatica ova (synonym Calodium hepatica) which were found with high frequency within granulomatous inflammation in the livers of trapped rats. Capillariasis was frequently observed within the epithelium of the squamous portions of the stomachs of rats trapped as part of this study.
C. hepatica has a direct life cycle which only requires a single host. The adult nematodes infect the liver of vertebrates and have been described in rodents, humans, pigs, a range of carnivores and non-human primates. Consumption of embryonated ova result in the larval worms migrating from the gastrointestinal tract into the liver. After a period of development which typically requires approximately 3 weeks, the mature females deposit large numbers of ova in the hepatic parenchyma, which elicit a chronic inflammatory response and granuloma formation.
Certain nematodes, such as Gongylonema neoplasticum (formerly Spiroptera carcinoma), have been implicated as being contributory to neoplastic transformation of gastric tissues. Furthermore, capillarid nematode infections in Norway rats examined in South America were noted to display hyperplastic changes in the non-glandular stomach. These changes were associated with posited infections with Eucoleus gastricus; formerly Capillaria gastrica; Hepaticola gastrica.16
In humans, hepatic capillariasis typically presents as an acute eosinophilic hepatitis with potential spread to distant viscera and in rare instances has been fatal.
Specific helminthic parasitism of rats in South East Asia has been described. One study of note involved a survey of rats in Northern Thailand in the moat surrounding the ancient city of Chiang Mai.13 In that instance, 38 Norway rats and roof rats (Rattus rattus) were trapped and necropsied during a 3 month period in 1995. From this sample set, 10 different helminths were identified infection the animals. Four species of trematode, to include Centrocestus sp., Echinostoma ilocanum, Echinostoma malayanum and Quinqueseralis quinqueseralis, two species of cestode, comprising Raillietina sp. And Taenia sp., as well as four species of nematode, which were Nippostrongylus sp. Angiostrongylus cantonensis, Rictularia sp. and the ova of Capillaria hepatica.
Similarly, a more robust study conducted in Malaysia examined the livers of over 100 rats specifically focusing on the two etiologies found in these slides. In that investigation, approximately 39% of rats were infected with C. fasciolaris and 45% with C. hepaticum, while 20% of all of the animals examined were noted to be parasitized by both etiologies.19
Contributing Institution:
Armed Forces Research Institute of Medical Sciences (AFRIMS). http://afrims.amedd.army.mil/usamd-afrims.html
JPC Diagnosis:
- Liver: Strobilocercus.
- Liver: Granuloma, focal, with numerous aphasmid eggs.
JPC Comment: The contributor has done an excellent job in the description of many aspects of the life cycle, epidemiology, and comparative pathology of the two etiologic agents in this case.
Recent investigations into the pathology of Cysticercus fasciolaris have denoted some new and interesting facts. It is apparent that C. fasciolaris is best adapted to the hepatic environment, where the cyst provides adequate protection from the host response. Cysts may be seen in other tissues, such as the kidney and abdominal wall, but the cysts are poorly formed and filled with degenerate neutrophils rather than a thriving stobilocercus. In the liver, the cyst will appears to go through a distinct maturation process in which collagen types I and III are demonstrable within the wall, with type III within the inner layers and type I in outer layers. During subsequent maturation of the cyst, type I collagen predominates. It is apparent that this staging and location of different types of collagen during development is required for prolonged survival of the parasite.11
Another feature of the cyst, mentioned above, is the rare but well-documented neoplastic cyst wall. First identified by Borrel in 1906, the hepatic sarcomas associated with the cyst wall of C. fasciolaris were first experimentally induced by Bullock and Rohdenburg in 1920, in which 25% of young rats developed sarcomas over a period of eight to fifteen months (occasionally not associated with parasites), and documented metastasis in 60% of tumors. The inability of other researchers to induce similar tumors suggests a variation in susceptibility of rat strains to develop this neoplasm.12
Capillaria hepatica has been identified in a number of species other than rodents which are the natural host, including cases in mammalian families Insectivora, Chiroptera, Lagomorpha, Artiodactyla, Perissodactyla, Hyracoidea, Marsupalia, Carnivora, and various primates, to include man.15 163 cases of human infection have been documented, to include true infections as well as spurious ones, in which unembryonated non-infective eggs are ingested and passed through the gastrointestinal tract.15 The disease in humans is similar to those seen in other aberrant mammalian hosts, with granuloma formation and marked hepatic fibrosis. Another related species, C. philippinensis, results in jejunal infections with mucosal invasion by the parasite. A recent report of capillariasis of C. hepatica infection in the horse, detailed partially calcified granulomas containing eggs were identified in the hepatic parenchyma.15
The moderator discussed the primary difference between a strobilocercus and a cysticercus, and that the neck and scolex is generally everted in tissue section with strobilocerci. The strobilocercus is generally considered a more advanced form than a cysticercus, as it more rapidly becomes an adult cestode after ingestion by the definitive host. The calcareous corpuscles in the strobilocerci were also discussed – in strobilicerci, these structures are present in the scolex and neck but not in the bladder; when pressed on the function of these structures, the moderator pointed out that it has never been definitively proven, but it was his belief that their large amount of calcium may be needed by the parasite as a buffer in the acidic nature of the enteron.
References:
- Chaiyabutr N. Hepatic capillariasis in Rattus norvegicus. J Sci Soc Thailand. 1979; 5:49-50.
- Chaisiri K, Chaeychomsri W, Siruntawineti J, Riba A, Herbreteau V and Morand S. Gastrointestinal Helminth Infections in Asian House Rats (Rattus tanezumi) from Northern and Northeastern Thailand. J Trop Med Parasitol. 2010;33:29-35.
- Claveria FG, Causapin J, de Guzman MA, Toledo MG and Salibay C. Parasite biodiversity in Rattus spp caught in wet markets. Southeast Asian J Trop Med Public Health. 2005;36 Suppl 4:146-8.
- Conlan JV, Sripa B and Attwood S, Newton PN. A review of parasitic zoonoses in a changing Southeast Asia. Vet Parasitol. 2011;182:22-40.
- Gardiner CH and Poynton SL. An Atlas of Metazoan Parasites in Animal Tissues. Washington, DC: American Registry of Pathology; 1999:40-43
- Gardiner CH and Poynton SL. An Atlas of Metazoan Parasites in Animal Tissues. Washington, DC: American Registry of Pathology; 1999:50-55.
- Himsworth CG, Parsons KL, Jardine C and Patrick DM. Rats, Cities, People, and Pathogens: A Systematic Review and Narrative Synthesis of Literature Regarding the Ecology of Rat-Associated Zoonoses in Urban Centers. Vector-Borne Zoonot. 2013; 13(6):349-359.
- Jittapalapong S, Herbreteau V, Hugot JP, Arreesrisom P, Karnchanabanthoeng A, Rerkamnuaychoke W and Morand S. Relationship of Parasites and Pathogens Diversity to Rodents in Thailand. Kasetsart J (Nat Sci). 2009; 43:106-117.
- Joshi PP. Human trypanosomiasis in India: Is it an emerging new zoonosis? Section 1: infectious diseases. Med Update. 2013; 23:10-13
- Kamiya M, Ooi HK, Ohbayashi M and Ow-Yang CK. Bicephalic larval cestode of Taeniidae from rats in Malaysia. Jap J Vet Res. 1987; 35(4):275-282.
- Lee BW, Jeon BS, Kim HS, Kim HC, Yoon BI. Custicercus fasciolaris infection in wild rats (Rattus norvegicus) in Korea and formation of cysts by remodeling of collagen fibers. J Vet Diagn Investig 2016; 28(3):263-270.
- Letich, A. The experimental inquiry into the causes of cancer. Brit Med J 1923; 2(3262):1-7.
- Linardi PM and Botelho JR. Prevalence of Trypanosoma lewisi in Rattus norvegicus from Belo Horizonte, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2002; 97(3):411-414.
- McCarthy J and Moore TA. Emerging helminth zoonoses. Int J Parasitol. 2002; 30:1351-1360.
- Ochi A, Hifumi T, Ueno T, Katayama Y. Capillaria hepatica (Calodium hepaticum) infection in a horse: a case report. BMC Vet Res 2017; 13:384-386.
- Namue C and Wongsawad C. A survey of helminth infection in rats (Rattus spp.) from Chaing Mai moat. Southeast Asian J Trop Med Public Health. 1997;28 Suppl 1:179-83.
- Rothenburger JL, Himsworth CG, Lejeune M, Treuting PM, Leighton FA. Lesions associated with Eucoleus sp. in the non-glandular stomach of wild urban rats (Rattus norvegicus). International Journal for Parasitology: Parasites and Wildlife. 2014;3(2):95-101.
- Sarataphan N, Vongpakorn M, Nuansrichay B, Autarkool N, Keowkarnkah T, Rodtian P, Stich RW and Jittapalapong S. Diagnosis of a Trypanosoma lewisi -like (Herpetosoma) infection in a sick infant from Thailand. J Med Microbiol. 2007; 56:1118–1121.
- Sinniah B, Narasiman M, Habib S, Gaik Bei O. Prevalence of Calodium hepaticum and Cysticercus fasciolaris in Urban Rats and Their Histopathological Reaction in the Livers. J Vet Med. 2014; 2014:172829.
- Stoj?evi D, Marinculi? A and Mihaljevi? Z. Prevalence of Capillaria hepatica in Norway rats (Rattus norvegicus) in Croatia. Veterinarski Archiv. 2002; 72(3):141-149.
- Sumangali K, Rajapakse RPVJ and Rajakaruna RS. Urban rodents as potential reservoirs of zoonoses: a parasitic survey in two selected areas in Kandy district. Ceylon J Sci (Bio Sci). 2012; 41(1):71-77.
- Taenia taeniaeformis. American Association of Veterinary Parasitologists (AAVP) http://www.aavp.org/wiki/cestodes/cyclophyllidea/taeniidae/taenia-taeniaeformis/
Accessed 14 February 2018