Signalment:
Gross Description:
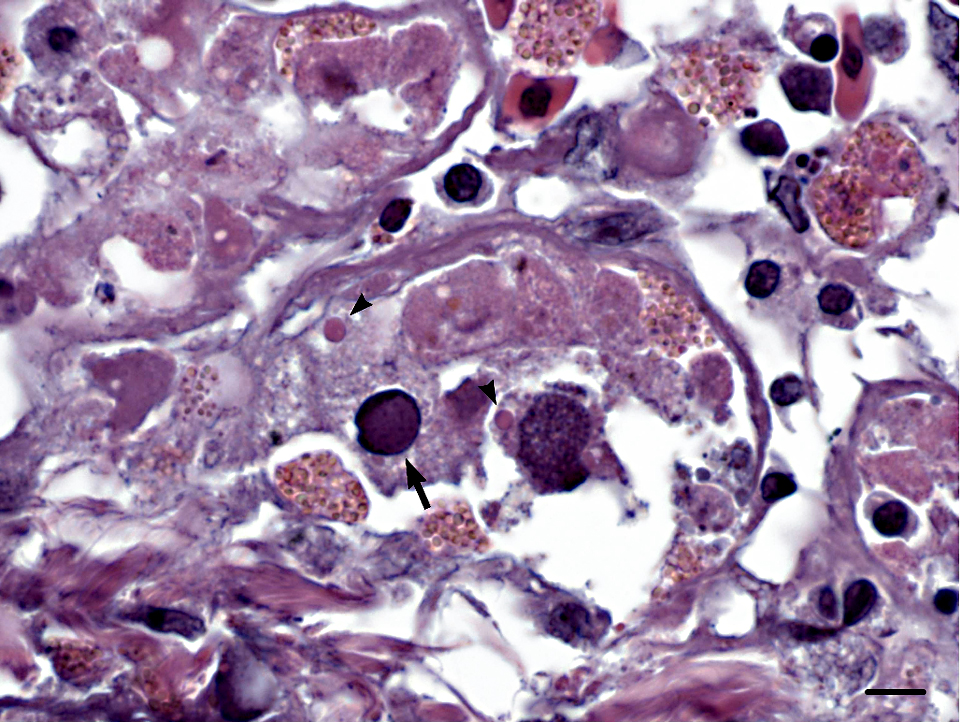
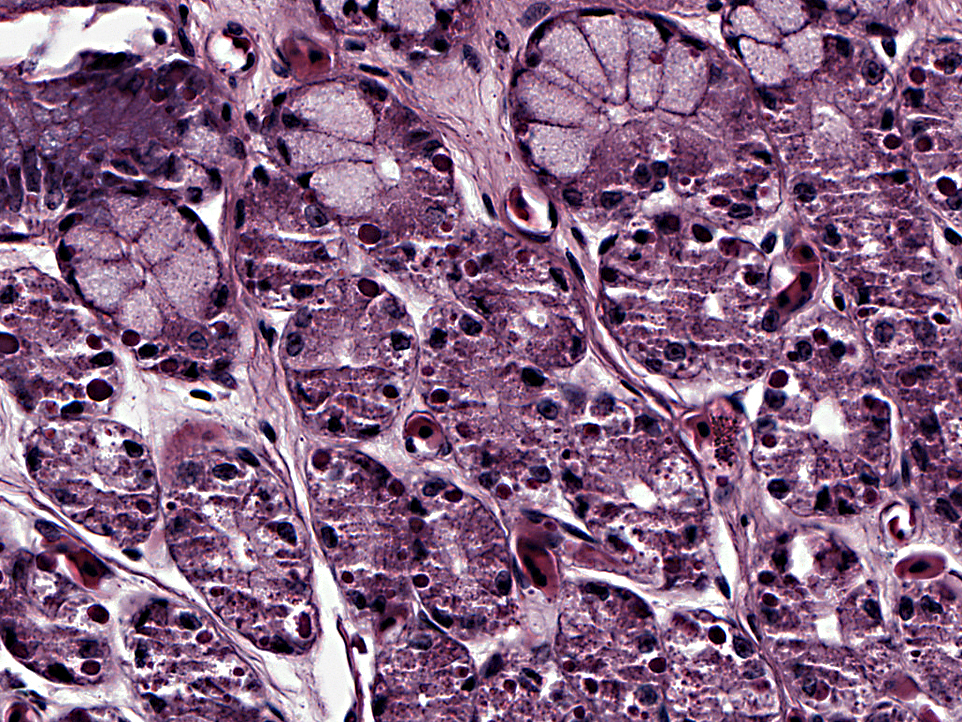
Histopathologic Description:
Liver: There is widespread hepatocellular necrosis associated with large, amorphic, amphophilic to basophilic intranuclear inclusion bodies resulting in karyomegaly and margination of nuclear chromatin. Occasional hepatocytes and numerous biliary epithelial cells contain one or more rounded, brightly eosinophilic intracytoplasmic inclusion bodies. Loose fibrin thrombi, high numbers of heterophils and scattered lymphocytes are present within sinusoids throughout the liver.
Stomach: Numerous mucosal epithelial cells contain rounded, brightly eosinophilic intracytoplasmic inclusion bodies. Occasional epithelial cells contain amphophilic intranuclear inclusion bodies. Additional lesions not included on the slide include brightly eosinophilic intracytoplasmic inclusion bodies within neurons and glial cells in the brain (occasionally associated with gliosis); within epithelial cells of the adrenal gland, thyroid follicles, bronchi, renal tubules, acinar pancreatic cells, and intestinal enterocytes; within lymphocytes in the spleen; and occasionally within cardiomyocytes. Low numbers of intestinal enterocytes also contain amphophilic intranuclear inclusion bodies.
Morphologic Diagnosis:
Liver: Severe, diffuse acute necrotizing hepatitis with intralesional inclusion bodies.
Stomach: Intracytoplasmic and intranuclear inclusion bodies.
Lab Results:
Condition:
Contributor Comment:
Inclusion body disease is an important infection in boid snakes, having emerged during the last 30 years on several continents.(13) Although snakes may remain chronic carriers of the disease, once clinical signs develop, the disease is fatal within weeks or months.(2,13) Clinical signs are variable and can include neurologic, digestive or respiratory systems.(6,12,13) Antemortem diagnosis can be achieved by biopsy of the liver, esophageal tonsil, gastric mucosa or skin; or by detection of typical cytoplasmic inclusion bodies within peripheral blood leukocytes, although this method yields higher false positives and false negatives.(2,3) Histologically, there are large, brightly eosinophilic intracytoplasmic inclusion bodies within a wide range of tissues (particularly in the liver and pancreas), often in the absence of a tissue or cellular response.(1,2,6,14)
Retroviruses have been isolated from boids with inclusion body disease; however, Kochs postulates have yet to be completely fulfilled.(5,13,16) The bloodsucking snake mite, Ophionyssus matrices, may act as a vector.(13) Concurrent infectious disease is common, possibly due to lymphoid depletion and resultant immunosuppression.(6) Pneumonia, ulcerative stomatitis(6), entamoebic colitis(10), and neoplastic diseases such as lymphoma(11) have been reported in conjunction with inclusion body disease.
Adenoviral hepatitis has previously been described in boid snakes, and is characterized by severe necrotizing hepatitis with basophilic to eosinophilic intranuclear inclusions within hepatocytes, accompanied by heterophils and small mononuclear cells.(4,8,13) Other lesions previously attributed to adenovirus or adenoviral-like infection in snakes include enteritis, splenitis, nephritis, pneumonia and encephalopathy.(7), The ability for latent adenoviral infections to induce clinical disease following immunosuppression has been demonstrated in species other than snakes(15), and in the case presented here it is tempting to speculate that inclusion body disease was involved in that respect.
| Major viral diseases in boid snakes | ||
| Agent | Typical lesions | Inclusions |
| Adenovirus | Necrotizing hepatitis | Amphophilic (occasionally eosinophilic), intranuclear |
| Herpesvirus | Necrotizing hepatitis, glomerulonephritis | Eosinophilic to amphophilic intranuclear inclusions |
| Inclusion body disease virus (suspected retrovirus) | Inclusions in the absence of a tissue or cellular response | Eosinophilic, intracytoplasmic |
| Paramyxovirus | Hemorrhagic to necrotizing pneumonia, pancreatitis, occasionally CNS disease | Occasional, eosinophilic, intranuclear or cytoplasmic |
| Adenovirus, parvovirus, picornavirus, herpesvirus | Viral-associated gastrointestinal disease - causal relationship not established | |
JPC Diagnosis:
1. Liver: Hepatic atrophy, diffuse, severe.
2. Liver: Necrosis, hepatocellular, multifocal, with rare intranuclear viral inclusions.
3. All cell types: Intracytoplasmic inclusions.
4. Stomach: Diffuse loss of parietal cell granules.
Conference Comment:
The moderator discussed the composition of the inclusions seen in inclusion body disease, which remains controversial. The inclusion material was initially thought to result from the associated retrovirus, but recent research suggests that this condition may be a storage disease, similar to the transmissible spongiform encephalopathies. The intracytoplasmic inclusions, which ultrastructurally are either non-membrane limited aggregates of granular electron-dense material, or membrane-bound aggregates of electron-dense material with membrane-like fragments, are composed of a distinct 68-kd protein, and typically cause no inflammatory response, as opposed to necrosis and inflammation associated with viral infections. Nonviral inclusions have been demonstrated in cells infected with other retroviruses, such as avian leukosis virus, visna virus, and simian immunodeficiency virus.
Boid inclusions stain with PTAH and toluidine blue, and are eosinophilic with H&E, suggesting protein composition; they do not stain with Congo red or thioflaven-T, suggesting that the material in not amyloid. Boid inclusions have a similar staining pattern to Mallory bodies, non-viral inclusions composed of cytokeratin filaments. The inclusions may also be overproduced or poorly degraded components of the virus that accumulates in the host cell cytoplasm.(16)
As this is one of the most important viral diseases of boas and pythons with no known treatment, diagnosis of IBD is imperative. Antemortem diagnosis is often easily obtained through biopsy, since the inclusions are present in all tissues. Preferring the liver, the moderator discussed various high-yield biopsy locations. The skin is a poorer option because inclusions are more widely distributed, necessitating multiple biopsies to decrease false positive results. Cytology is another option, but the slides must be stained with H&E in order to differentiate the inclusions from other structures, such as lipid or intracellular parasites. Currently, immunohistochemical staining is available for frozen tissue, which may lead to a serologic test in the future.
References:
2. Chang LW, Jacobson ER. Inclusion body disease, a worldwide disease of boid snakes: A review. J Exotic Pet Med. 2010;19:216-225.
3. Garner MM. Methods for diagnosing inclusion body disease in snakes. In: Small animal and exotics. Proceedings of the North American Veterinary Conference. 2005;19:1283-1284.
4. Jacobson ER, Gaskin JM, Gardiner CH. Adenovirus-like infection in a boa constrictor. JAVMA. 1985;187:1226-1227.
5. Jacobson ER, Or³s J, Tucker SJ, et al. Partial characterization of retroviruses from boid snakes with inclusion body disease. AJVR. 2001;62:217-224.
6. Or³s J, Tucker S, Jacobson ER. Inclusion body disease in two captive boas in the Canary Islands. Vet Rec. 1998;143:283-285.
7. Perkins LEL, Campagnoli RP, Harmon BG, et al. Detection and confirmation of reptilian adenovirus infection by in situ hybridization. J Vet Diagn Invest. 2001;13:365-368.
8. Ramis A, Fern+â-índez-Bellon H, Maj³ N, et al. Adenovirus hepatitis in a boa constrictor (Boa constrictor). J Vet Diagn Invest. 2000;12:573-576.
9. Raymond JT, Lamm M, Nordhausen R, et al. Degenerative encephalopathy in a coastal mountain kingsnake (Lampropeltis zonata multifasciata) due to adenoviral-like infection. J Wild Dis. 2003;39:431-436.
10. Richter B, K+â-+bber-Heiss A, Weissenb+â-¦ck H. Diphtheroid colitis in a Boa constrictor infected with amphibian Entamoeba sp. Vet. Parasit. 2008;153:164-167.
11. Schilliger L, Selleri P, Frye FL. Lymphoblastic lymphoma and leukemic blood profile in a red-tail boa (Boa constrictor) with concurrent inclusion body disease. J Vet Diagn Invest. 2011;23:159-162.
12. Schumacher J, Jacobson ER, Burns R, et al. Adenovirus-like infection in two rosy boas (Lichanura trivirgata). J Zoo Wild Med. 1994;25:461-465.
13. Schumacher J, Jacobson ER, Homer BL, et al. Inclusion body disease in boid snakes. J Zoo Wild Med. 1994;25:511-524.
14. Vancraeynest D, Pasmans F, Martel A, et al. Inclusion body disease in snakes: a review and description of three cases in boa constrictors in Belgium. Vet Rec. 2006;158:757-761.
15. Ward JM, Young DM. Latent adenoviral infection of rats: intranuclear inclusions induced by treatment with a cancer chemotherapeutic agent. JAVMA. 1976;169:952-953.
16. Wozniak E, McBride J, DeNardo D, et al. Isolation and characterization of antigenically distinct 68-kd protein from nonviral intracytoplasmic inclusions in boa constrictors chronically infected with the inclusion body disease virus (IBDV: Retroviridae). Vet Pathol. 2000;37:449-459.