WSC 22-23
Conference 1
CASE II:
Signalment:
32-month-old, female, cross- corriedale sheep (Ovis aries)
History:
This ewe showed depression, dysphagia, nasal discharge, coughing, and drooling. Two days prior to death, she developed a high fever, constant salivation and head drooping. There were no vesicular formation or ulcers in the oral cavity. Subsequently, the sheep died, and was autopsied in the next morning. No animals had been introduced on this farm for three years.
Gross Pathology:
The lung presented bilaterally dark red in color with a firm consistency and did not collapse after the thorax was opened. The respiratory tract was filled with yellowish-white foamy materials. The gastrointestinal tract was dark red in color throughout.
Laboratory Results:
The gene of Bluetongue virus (serotype 21) was detected from the blood samples by reverse transcription-polymerase chain reaction (RT-PCR).
Any bacterial pathogens were not detected from major organs examined. Postmortem testing of hepatic selenium and Vitamin E concentrations reveal levels as adequate.
Microscopic Description:
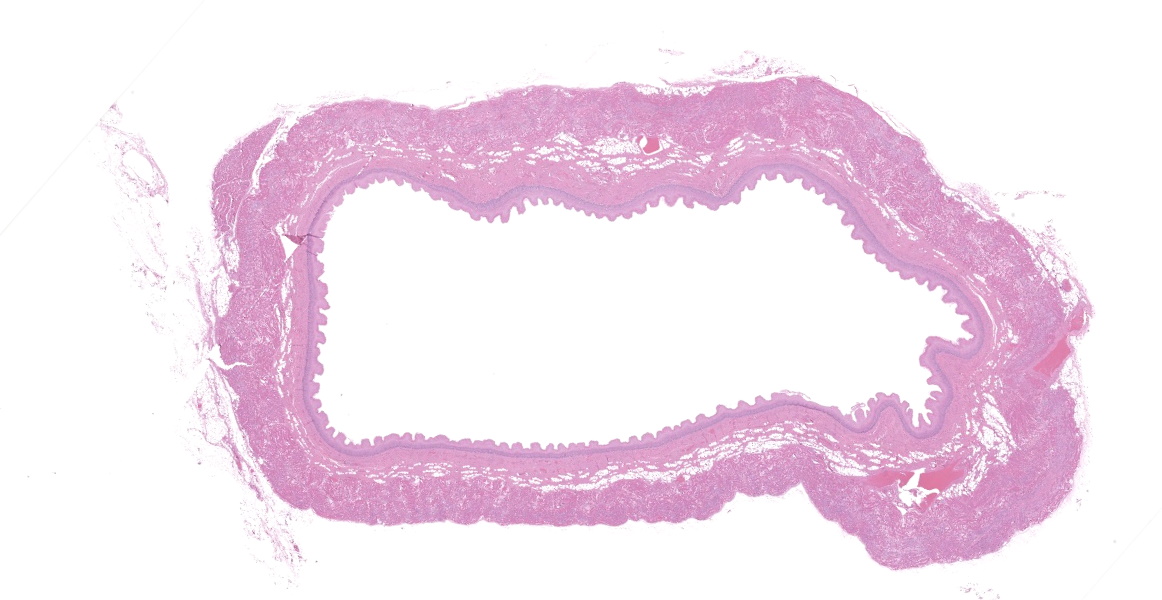
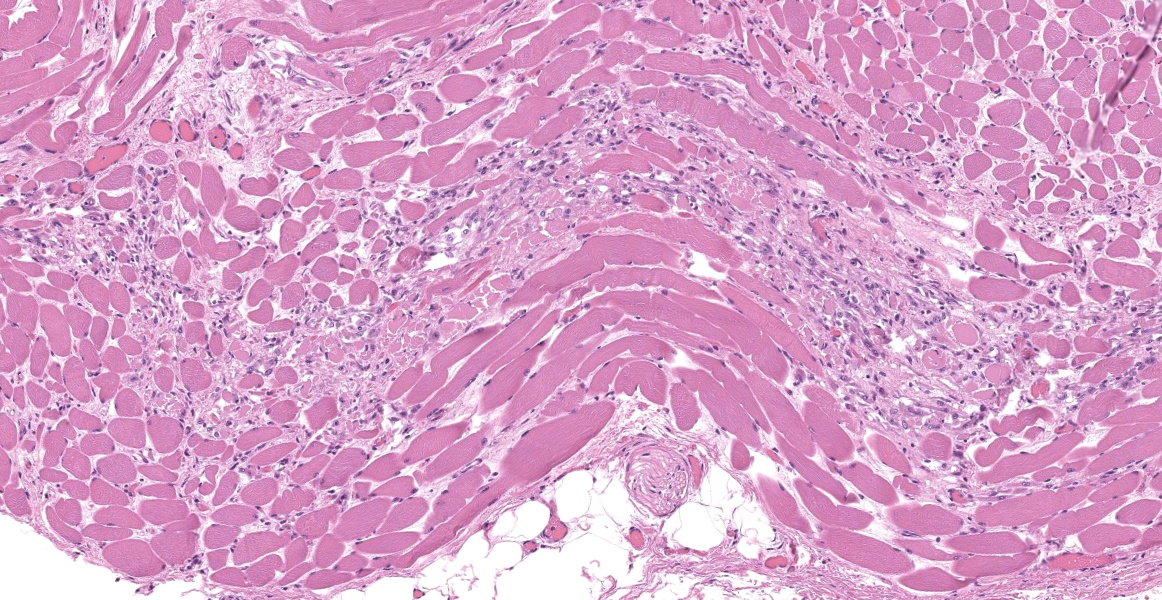
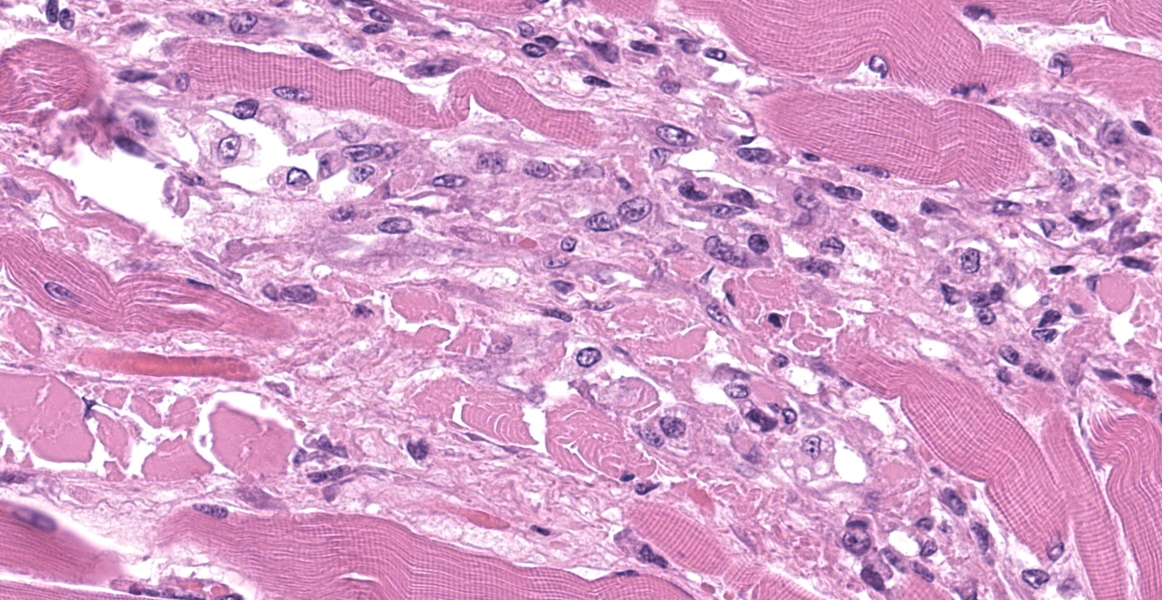
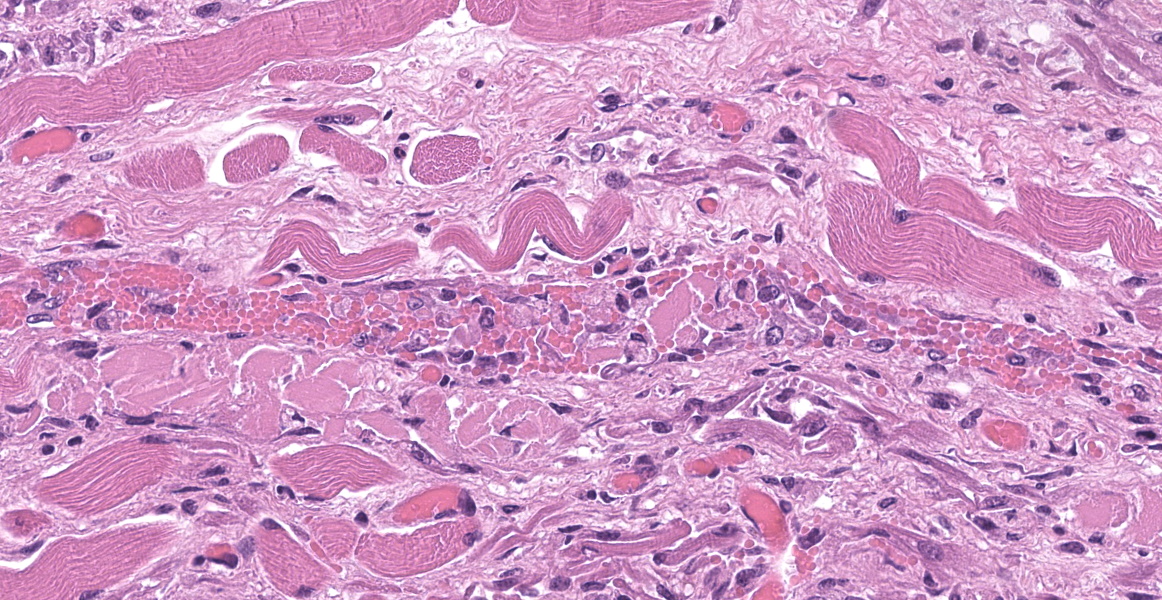
Upper esophagus: The histologic lesion is mainly in the muscle layer as multifocal muscle fiber degeneration and necrosis, and the epithelial layer is intact. Degenerate and necrotic muscle fibers are fragmented with loss of cross striations, and hyalinized homogenous sarcoplasm. There is infiltration of prominent macrophages, a few lymphocytes and plasma cells between affected muscle fibers. The necrotic areas are replaced by a variable amount of collagens and fibroblasts. The regenerative change of myofibers characterized by a row of nuclei, centralized nuclei and basophilic cytoplasm frequently appears adjacent to affected region. Small blood vessels are occasionally lined by hypertrophied endothelial cells or disrupted endothelium with scattered pyknotic or karyorrhectic nuclei.
Contributor’s Morphologic Diagnosis:
Esophagus: Multifocal myofiber degeneration and necrosis, moderate, with mild fibrosis and regeneration.
Contributor’s Comment:
This slide presents a typical case of Bluetongue (BT) with muscle fiber degeneration and necrosis, and a vascular lesion. The muscle lesion also showed regenerative changes including basophilic myoblasts, myotubes and fibrovascular response. These findings indicate that 1-2 weeks have elapsed since lesion formation. This is consistent with that the ewe died about a week after the onset of clinical symptoms.
BT is an infectious, non-contagious, vector-borne viral disease that affects wild and domestic ruminants.14 The disease is caused by Bluetongue virus (BTV), family Reoviridae, the prototype virus for genus Orbivirus.1 At least 27 or more distinct BTV serotypes were defined.4,12 The virus is essentially transmitted by Culicoides biting midges among susceptible animals, however vertical transmission and horizontal transmission have also been reported. 2,13,16,21 BTV infection has been reported from worldwide including tropical, subtropical and some temperate regions, and the global distribution of the virus is consistent with the distribution of competent insect vectors and appropriate climatic conditions.15
Among domestic ruminants, clinical BT cases mainly occur in sheep. Clinical manifestations of the disease vary widely depending on the type and strain of infected virus, rearing factors and breed. Other species including cattle and goat are typically asymptomatic or subclinical, although specific BTV strains such as serotype 1 and 8, currently circulating in Europe, can induce severe disease in these animals. 5,10,23 BT in sheep causes severe hemorrhagic syndrome characterized by fever, edema, hemorrhages, dyspnea, mucosal erosions and ulcerations, muscle necrosis, and coronitis.6,20These symptoms are generally reflect viral kinetics.
After Culicoides insect bites, BTV first replicates in the regional lymph nodes, then it spreads to other organs including lung, lymph nodes and spleen. BTV demonstrates a tropism for a variety of cell types, including dendritic cells, mononuclear phagocytic cells, activated lymphocytes and endothelial cells. 9,11,22 The virus replication in endothelial cells directory induce degenerate, necrotic, and hyperplasic changes in the endothelium, which result in increased of vascular permeability and causing edema, congestion, hemorrhage, thrombosis and necrosis. Additionally, the inflammatory and vasoactive mediators released by BTV infected cells have been proposed as responsible for worsening of endothelial dysfunction and vascular damage.14
Pulmonary lesions such as edema and hemorrhage are important change observed in almost all BT in sheep.14 Greater susceptibility to BTV replication is reported in endothelial cells of respiratory micro-vesicular system.7,8 Moreover, aspiration pneumonia is frequently observed as a result of aspiration of regurgitated food content followed by paralysis of the esophagus due to muscle necrosis induced by virus induced vascular damage.3
The differential diagnosis of BT in sheep should be done in conjunction with other diseases that cause edema, hemorrhage and epithelial damage.3 The type of lesion and its distribution in affected animals can support development of BT diagnosis. The differential diagnosis includes foot-and-mouth disease, vesicular stomatitis, peste des petits ruminants, contagious ecthyma, sheep pox and photosentisation.19,25 The initial lesion of BT is similar to foot-and-mouth disease. However, foot-and-mouth disease lesions are vesicular, whereas BT lesions are hemorrhagic and edematous. Vesicular stomatitis is also a disease that can be mistaken for BT. Since the lesion findings are similar to those of foot-and-mouth disease, the same criteria can be used to make a diagnosis. In the case of peste des petits ruminants, extensive gastrointestinal mucosal lesions are impressive, and may be concentrated in lymphoid tissues, such as Peyer's patches. In the case of contagious ecthyma and sheep pox, histopathology revealed proliferative lesion and intracytoplasmic inclusion body in epithelial layers. Photosensitization is also included in differential diagnosis. The absence of hemorrhage and erosions in the oral cavity, in addition to the generalized nature of the skin lesions, and their association with non-pigmented areas of the skin may be helpful for diagnosis. In addition, lesions such as myonecrosis in BT may mimic nutritional muscular dystrophy due to vitamin E and selenium deficiency. It is important that the clinical history and lesions be confined to the muscle for the differential diagnosis.
Contributing Institution:
National Institute of Animal Health,
National Agriculture and Food Research Organization (NARO)
3-1-5 Kannondai, Tsukuba, Ibaraki 3050856, Japan
JPC Diagnosis:
Esophagus: Myocyte degeneration, necrosis, and regeneration, circumferential, multifocal to coalescing, moderate, with replacement fibrosis and multifocal vasculitis.
JPC Comment:
The contributor provides an excellent overview of the pathogenesis, clinical signs, and differential diagnoses for bluetongue virus (BTV) infections.
While phylodynamic models estimate that BTV has been circulating in ruminants for over a millennium, the first documented cases occurred in South Africa in the nineteenth century, and in 1902, the term malarial catarrhal fever was used to describe the disease. Once a viral etiology was uncovered in 1905, the disease became known by its current and similarly descriptive name, which denotes the cyanosis most visible on the swollen tongue. The disease slowly spread through tropical climates during the first half of the twentieth century, and has now also been reported in Australia, the Americas, Asia, and Europe with serious economic consequences.4 Ongoing geographic spread of this arbovirus can be partially attributed to changing environmental conditions and subsequent expansion of the Culicoides vector habitat.17,18
BTV is a segmented dsRNA virus that encodes seven structural (VP 1-7) and five non-structural (NS 1-4) proteins.17 VP7 is the most conserved protein and the basis for most seroconversion assays.18 As the contributor describes, there are at least 27 serotypes of BTV, and this diversity is due to high variability of VP2, segment re-assortment during infection with multiple strains, and genetic drift.17,18 There is little cross-protection between serotypes.18 Recently, several atypical serotypes of BTV have been identified which are associated with asymptomatic to mild infections in small ruminants only and at least one of these serotypes (BTV-25) is associated with persistent infection.18
After initial infection, the BTV causes a biphasic viremia, with the initial spike in viral replication reduced by an interferon response.18 BTV affects multiple lymphoid populations early during viremia, producing transient immunosuppression and increased susceptibility to secondary infections.18 During the second and third week, adaptive immune responses begin effectively controlling infection with expansion of CD8+ T-cells and CD21+ B cells inducing seroconversion against VP7. In some cases, the virus may persist by evading these adaptive immune responses. BTV can disrupt the function of follicular dendritic cells, delaying antibody production by B cells and decreasing T cell responsiveness through unknown mechanisms.18 Ongoing research into BTV dynamics and immune evasion will be critical in understanding and controlling this important disease.
Conference participants discussed esopha-geal hyperkeratosis and potential causes in this case. Orthokeratotic hyperkeratosis in the esophagus can occur in an anorectic animal as keratinized squamous epithelial cells are not removed by passing ingesta.24 Alternatively, parakeratotic hyperkeratosis may occur with reactive epithelial hyperplasia from epithelial injury.24
References:
- Attoui H, Maan S, Anthony SJ, Mertens PPC. Bluetongue virus, other orbiviruses and other reoviruses: Their relationships and taxonomy. In: Bluetongue. 1st ed. London, UK: Academic Press; 2009:23–52.
- Backx A, Heutink R, van Rooij E, van Rijn P. Transplacental and oral transmission of wild-type bluetongue virus serotype 8 in cattle after experimental infection. Vet Microbiol. 2009;138:235–243.
- Bianchi RM, Panziera W, Faccin TC, et al. Clinical, pathological and epidemiological aspects of outbreaks of bluetongue disease in sheep in the central region of Rio Grande do Sul. Pesqui Vet Bras. 2017;37:1443–1452.
- Bumbarov V, Golender N, Jenckel M, et al. Characterization of bluetongue virus serotype 28. Transbound Emerg Dis. 2020;67:171–182.
- Dal Pozzo F, De Clercq K, Guyot H, et al. Experimental reproduction of bluetongue virus serotype 8 clinical disease in calves. Vet Microbiol. 2009;136:352–358.
- Darpel KE, Batten CA, Veronesi E, et al. Clinical signs and pathology shown by British sheep and cattle infected with bluetongue virus serotype 8 derived from the 2006 outbreak in northern Europe. Vet Rec. 2007;161:253–261.
- DeMaula CD, Jutila MA, Wilson DW, MacLachlan NJ. Infection kinetics, prostacyclin release and cytokine-mediated modulation of the mechanism of cell death during bluetongue virus infection of cultured ovine and bovine pulmonary artery and lung microvascular endothelial cells. J Gen Virol. 2001;82:787–794.
- DeMaula CD, Leutenegger CM, Jutila MA, MacLachlan NJ. Bluetongue virus-induced activation of primary bovine lung microvascular endothelial cells. Vet Immunol Immunopathol. 2002;86:147–157.
- Drew CP, Heller MC, Mayo C, Watson JL, MacLachlan NJ. Bluetongue virus infection activates bovine monocyte-derived macrophages and pulmonary artery endothelial cells. Vet Immunol Immunopathol. 2010;136:292–296.
- Fabiana DP, Claude S, Etienne T. Bovine infection with bluetongue virus with special emphasis on European serotype 8. Vet J. 2009;182:142–151.
- Hemati B, Contreras V, Urien C, et al. Bluetongue Virus Targets Conventional Dendritic Cells in Skin Lymph. J Virol. 2009;83:8789–8799.
- Jenckel M, Emmanuel B, Schulz C, et al. Complete coding genome sequence of putative novel bluetongue virus serotype 27. Genome Announc. 2015;3(2):e00016-15.
- Koenraadt CJM, Balenghien T, Carpenter S, et al. Bluetongue, Schmallenberg - what is next? Culicoides-borne viral diseases in the 21st century. Vol. 10, BMC Vet Res. 2014;10:77.
- Maclachlan NJ, Drew CP, Darpel KE, Worwa G. The Pathology and Pathogenesis of Bluetongue. J Comp Pathol. 2009;141:1–16.
- Maclachlan NJ, Mayo CE, Daniels PW, Savini G, Zientara S, Gibbs EPJ. Bluetongue. OIE Rev Sci Tech. 2015;34:329–340.
- Menzies FD, McCullough SJ, McKeown IM, et al. Evidence for transplacental and contact transmission of bluetongue virus in cattle. Vet Rec. 2008;163:203–209.
- Rivera NA, Varga C, Ruder MG, et al. Bluetongue and Epizootic Hemorrhagic Disease in the United States of America at the Wildlife-Livestock Interface. Pathogens. 2021;10: 1-21.
- Rodriguez-Martin D, Louloudes-Lazaro A, Avia M, Martin V, Rojas JM, Sevilla N. The Interplay between Bluetongue Virus Infections and Adaptive Immunity. Viruses. 2021;13: 1-17.
- Rojas JM, Rodríguez-Martín D, Martín V, Sevilla N. Diagnosing bluetongue virus in domestic ruminants: current perspectives. Vet Med Res Reports. 2019;10:17–27.
- Schulz C, Sailleau C, Bréard E, et al. Experimental infection of sheep, goats and cattle with a bluetongue virus serotype 4 field strain from Bulgaria, 2014. Transbound Emerg Dis. 2018;65:e243–e250.
- Sluijs MTW van der, Schroer-Joosten DPH, Fid-Fourkour A, et al. Transplacental transmission of bluetongue virus serotype 1 and serotype 8 in sheep: Virological and pathological findings. PLoS One. 2013; 8(12): e81429.
- Stott JL, Blanchard-Channell M, Scibienski RJ, Stott ML. Interaction of bluetongue virus with bovine lymphocytes. J Gen Virol. 1990;71:363–368.
- Toussaint JF, Vandenbussche F, Mast J, et al. Bluetongue in northern Europe. Vet Rec. 2006;159:327.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie GM, ed. Vol. 2, Jubb, Kennedy, and Palmer’s pathology of domestic animals. St. Louis, Missouri: Elsevier, Inc; 2016:31.
- Williamson S, Woodger N, Darpel K. Differential diagnosis of bluetongue in cattle and sheep. In Pract. 2008;30:242–251.