CASE 2: 143/16 (4085104-00)
Signalment:
2-month-old, male foal, Brazilian Sport Horse, Equus caballus, equine.
History:
The horse was presented at the Veterinary Hospital of the Universidade Federal de Minas Gerais with left hind limb lameness and swelling of the left stifle joint. Few days after admission at the Veterinary Hospital, it developed recurrent fever, lethargy, increased respiratory rate, swelling of the left shoulder joint, and fluid diarrhea. Antibiotics and anti-inflammatory drugs were administered but no clinical improvement was observed.
Gross Pathology:
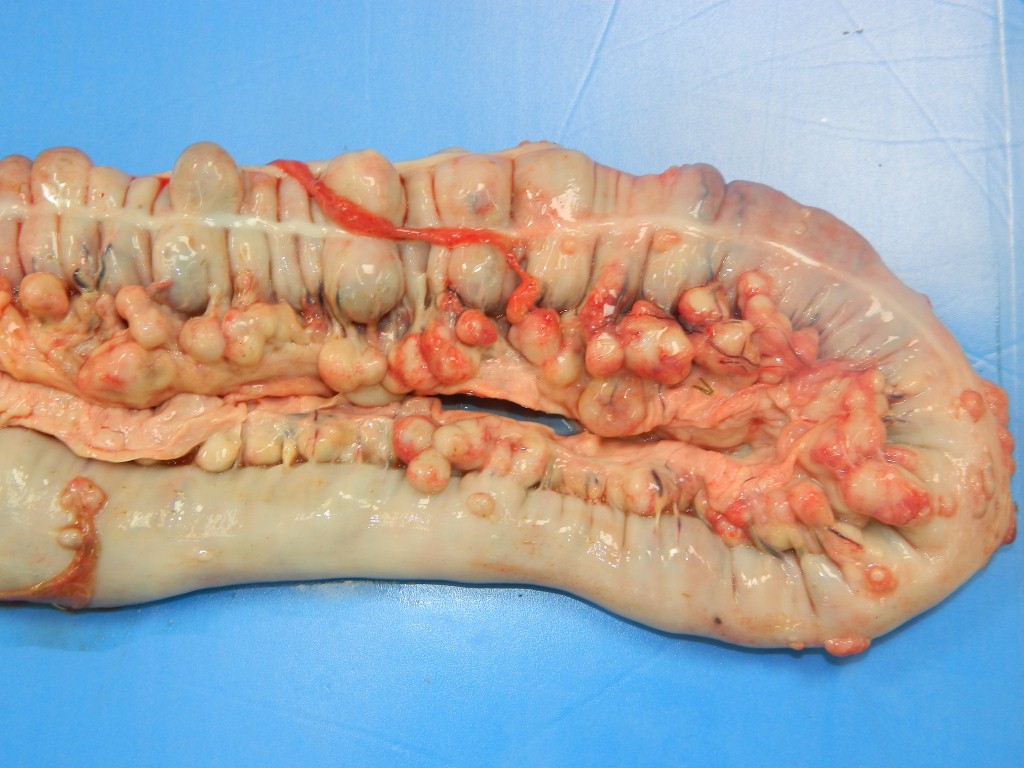
The foal was in good body condition. The stifle joint of the left pelvic limb was moderately swollen. The subcutaneous tissue over the joint was expanded by moderate amounts of a yellowish and viscous fluid (purulent exudate), which also extended to and dissected adjacent muscles. The superficial lymph nodes (axillar and cervical superficial) were moderately enlarged, and their parenchyma was replaced by white to yellow and soft exudate. The joint capsule was markedly thickened, and the articular cartilage was irregular. There was a moderate amount of a yellowish and viscous fluid within the stifle joint (purulent arthritis). Scattered throughout all lung lobes 1.0 to 9.0 cm in diameter white to yellow, slightly salient soft nodules were observed. On cut surface, moderate amount of a creamy yellow material (purulent exudate) oozed from the nodules (pyogranulomatous pneumonia). Large colon mucosa was irregular and thickened with salient areas admixed with 2.0 to 10.0 cm in diameter, whitish to grayish depressed areas (ulcers). Within the intestinal wall there were multiple small, 0.5 to 2.0 cm in diameter, white to yellow soft nodules. Cecal, colonic, mesenteric and tracheobronchial lymph nodes were markedly enlarged (5.0-10.0 cm in diameter), white to yellow and soft. Nodal cut surfaces were irregular and yellowish, with loss of the corticomedullary distinction, due to accumulation of variable amounts of a creamy yellow material (purulent exudate).
Laboratory results:
Laboratory results are pending.
Microscopic description:
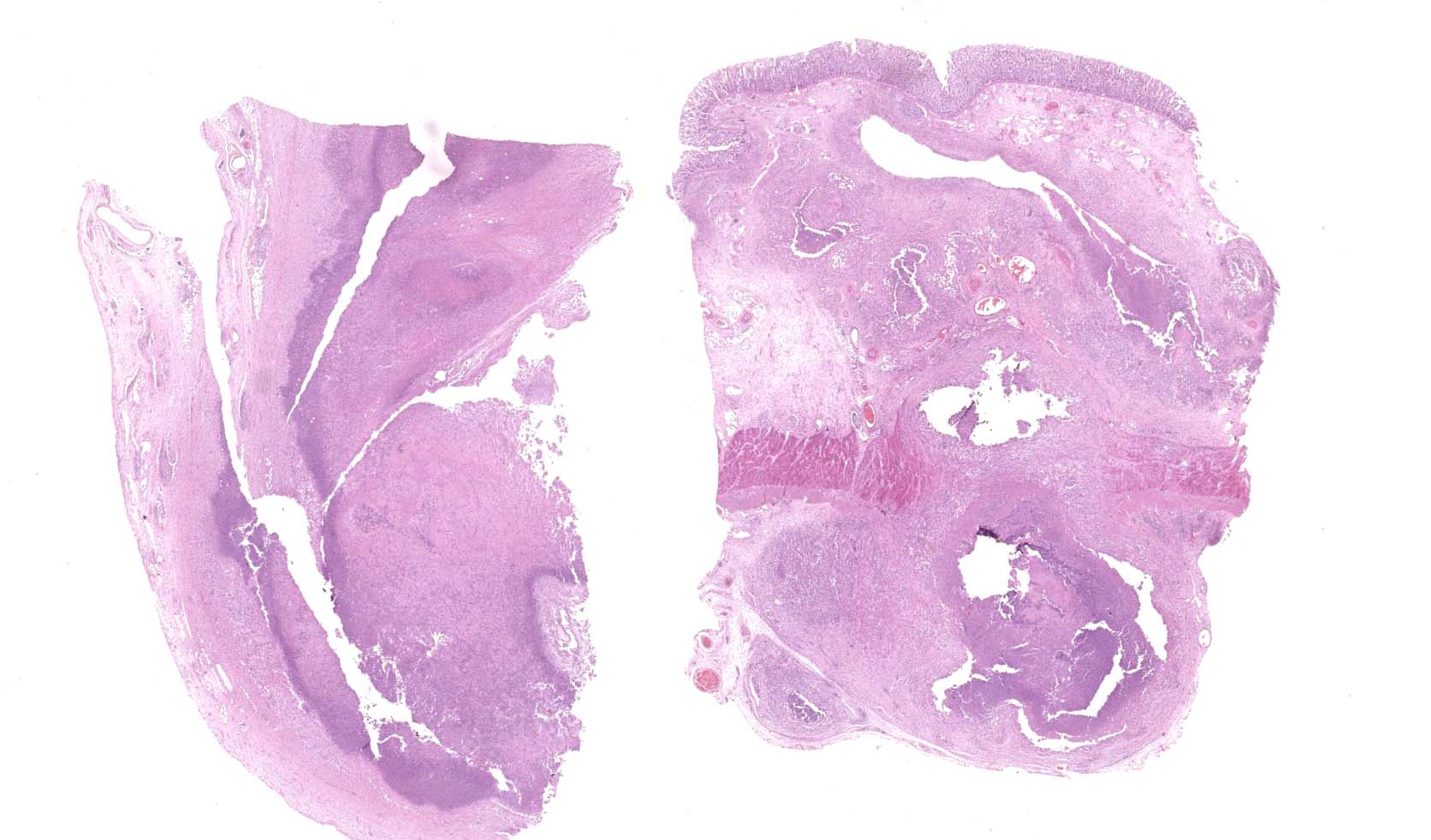
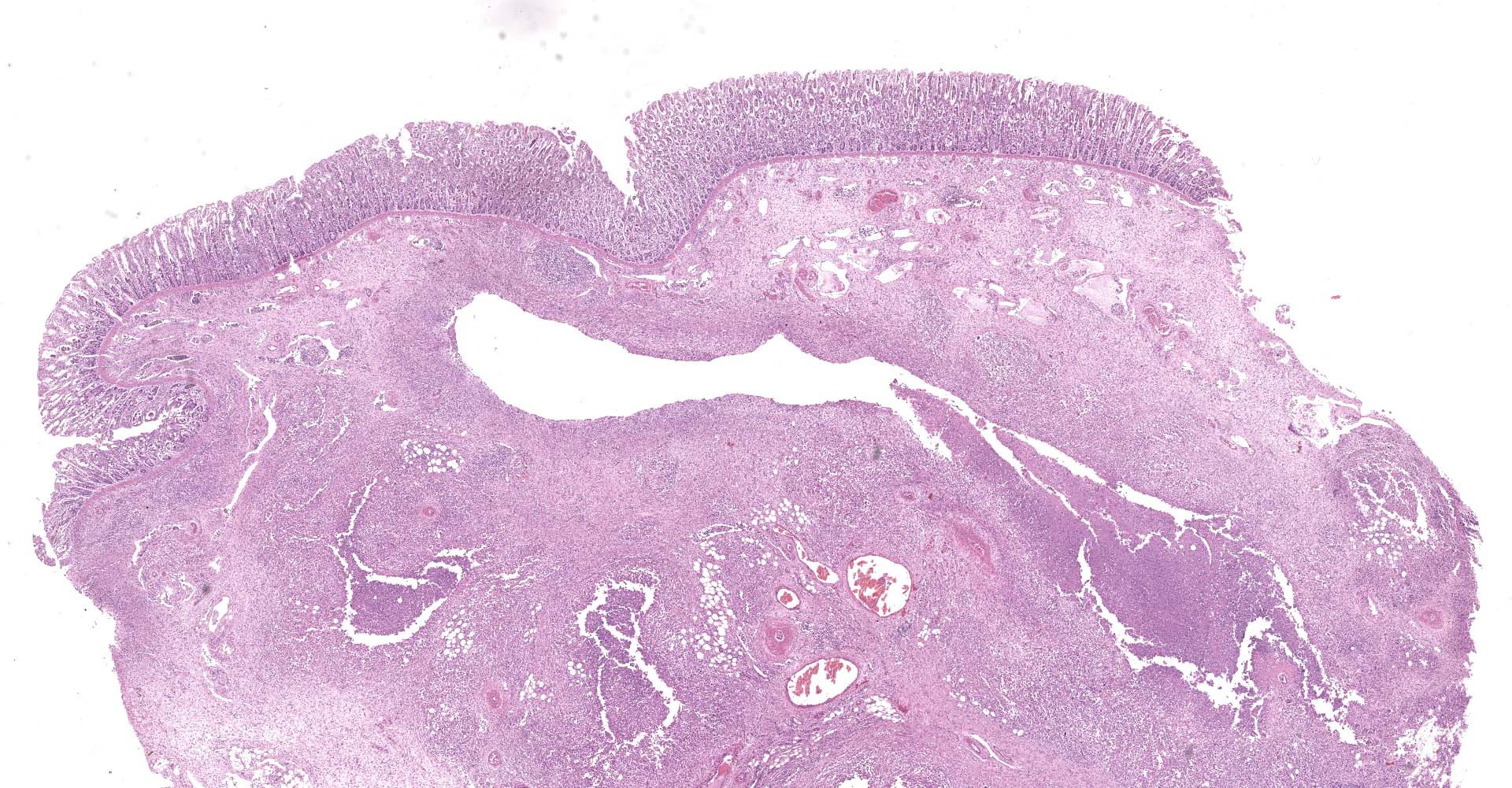
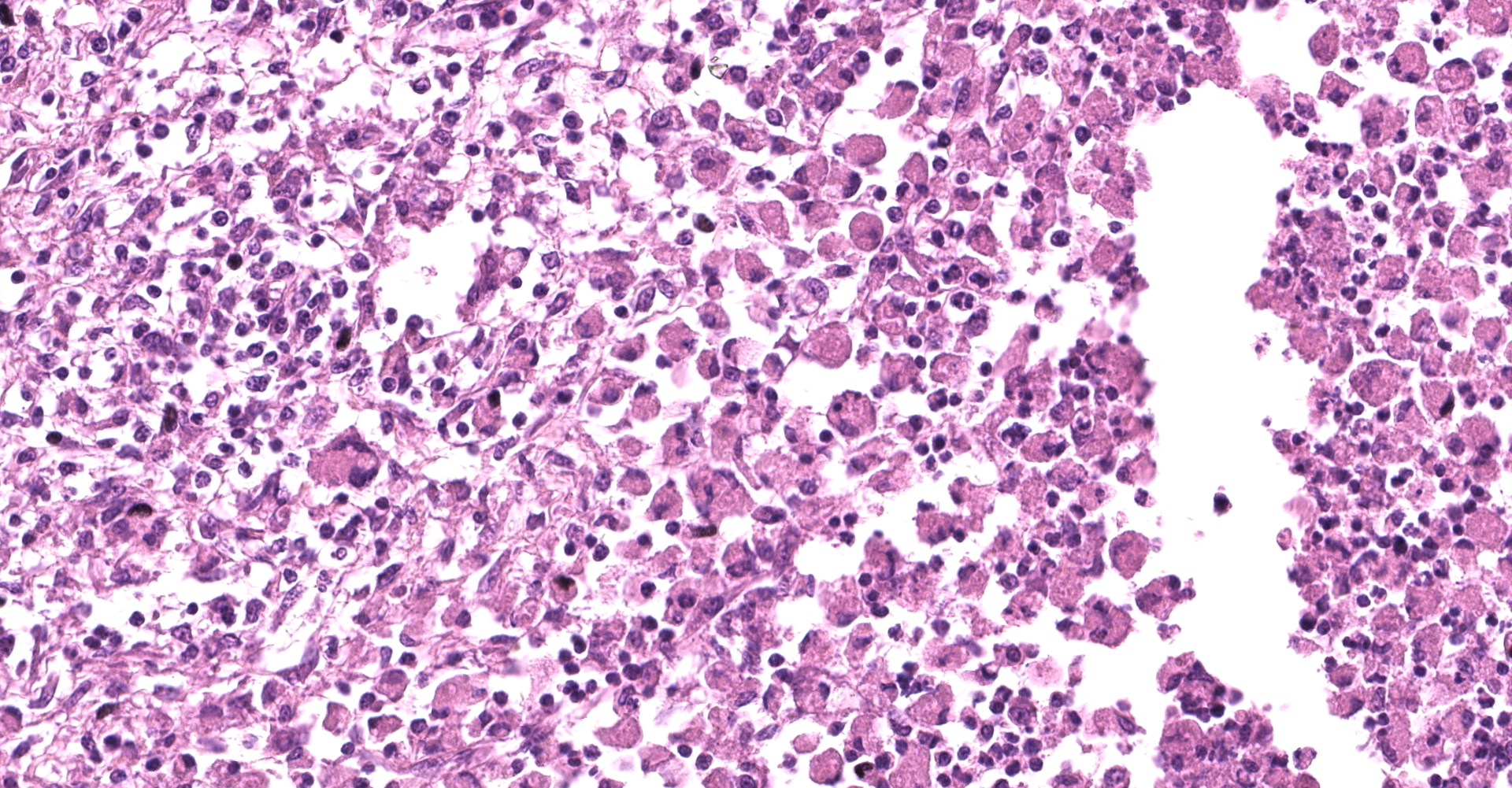
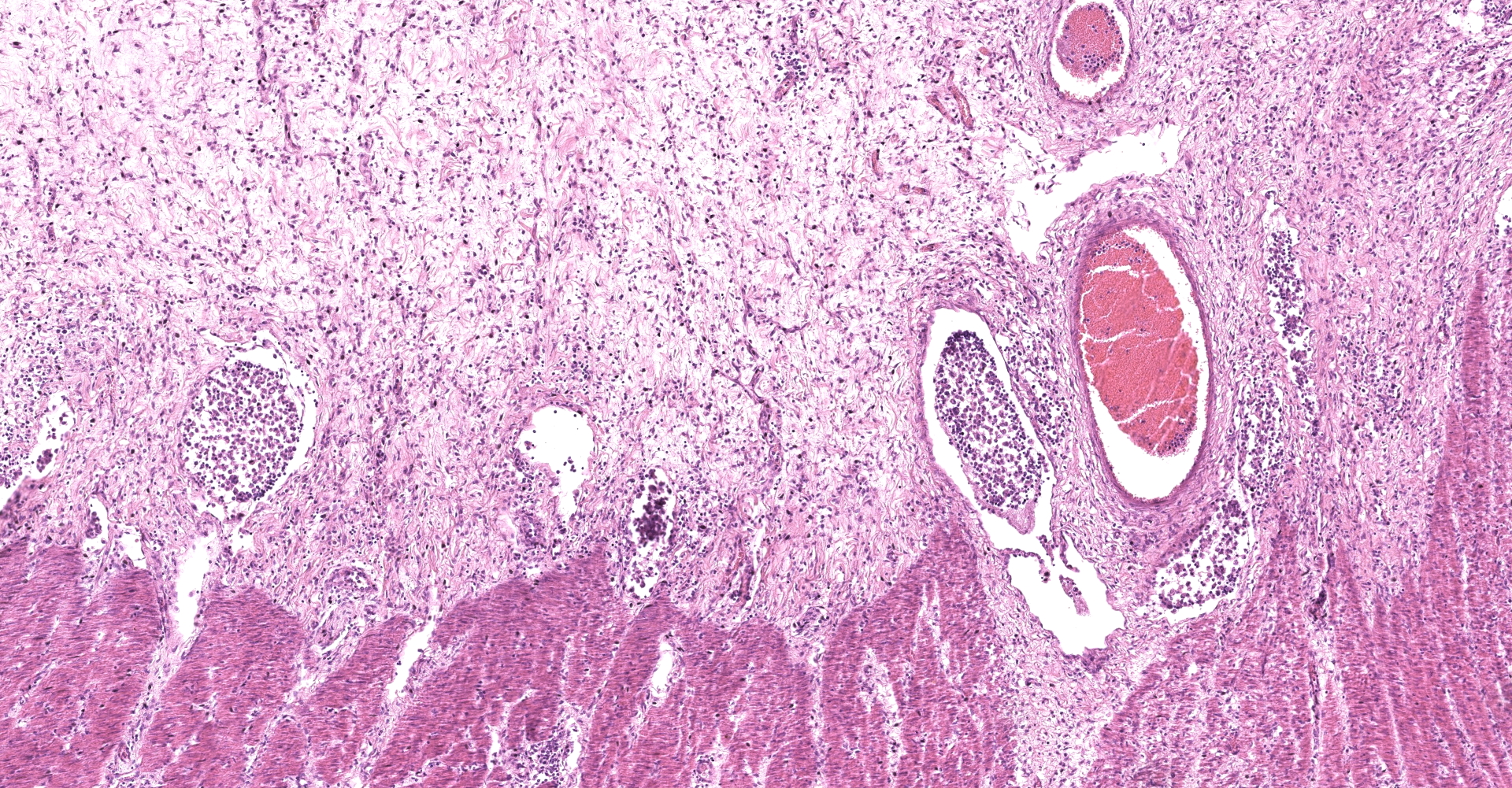
Large colon: locally extensive areas of the colonic submucosa, muscular layers and serosa are replaced and expanded by inflammatory nodules (pyogranulomas). These nodules are composed by a core of abundant eosinophilic cellular material and nuclear debris (necrosis), admixed with numerous degenerated and viable neutrophils, surrounded by a thick rim of moderate numbers of epithelioid macrophages and multinucleated giant cells (Langerhans type). Many of the macrophages contain intracytoplasmic bacteria (coccobacilli). These inflammatory nodules are further bounded by a thick layer of fibrous connective tissue admixed with numerous blood vessels (neovascularization - granulation tissue) infiltrated by moderate numbers of lymphocytes, plasma cells and macrophages. Collagen bundles in these areas and in the submucosa are separated by clear, non-staining spaces (edema). Several medium sized caliber blood vessels (arteries and veins) in the submucosa and in the serosa are partial to completely occluded by dense aggregates of an eosinophilic fibrillar material intermingled with small numbers of lymphocytes, neutrophils, erythrocytes and nuclear debris (fibrin thrombi). Rarely, the center of some thrombi is obscured by roughly granulated, hyperbasophilic and vitreous material (mineralization). Multifocally within the submucosa and serosa, there are prominent, dilated lymphatic vessels (lymphangiectasia), frequently filled with slight eosinophilic, fibrillar material (fibrin) with entrapped leukocytes. Few blood vessels present an irregularly shaped lumen, compressed by multifocal to diffuse subintimal deposits of granulated and hyperbasophilic material admixed with macrophages.
Mesenteric lymph node (per contributor): diffusely, nodal architecture is completely effaced and replaced by abundant eosinophilic cellular material and nuclear debris admixed with numerous degenerated and viable neutrophils. Surrounding this core are moderate numbers of epithelioid macrophages, occasional multinucleated giant cells (Langerhans type) and fewer lymphocytes and plasma cells. Rare macrophages and multinucleated giant cells contain intracytoplasmic bacteria (coccobacilli). Scattered within the cortical area and extending to the adjacent capsule, there are moderate numbers of fibroblasts along with collagen fibers (fibrosis). Rarely, in the cortical region, small aggregates of remaining lymphoid follicles are compressed (not present in all slides).
Tissues not submitted:
Lung present multiple pyogranulomas similar to those described in the intestine.
Contributor's morphologic diagnosis:
Large colon: Colitis, pyogranulomatous, multifocal to coalescing, transmural, marked, with fibrosis, thrombosis, lymphangiectasia and numerous intrahistiocytic coccobacilli
Mesenteric lymph node (per contributor): Lymphadenitis, pyogranulomatous, diffuse, marked, with rare intrahistiocytic coccobacilli
Contributor's comment:
The gross and histologic lesions in the lung, lymph nodes and intestine were typical of lesions caused by Rhodococcus equi. The confirmation of Gram-positive coccobacilli within the cytoplasm of macrophages corroborates the presumptive diagnosis. Laboratory results (molecular analysis) are still pending. Nevertheless, R. equi colonies were not observed in the first attempt to isolate this bacterium from lung and intestine samples.
R. equi is a facultative, intracellular, Gram-positive bacterium that preferentially infects macrophages.5 Virulent (and avirulent) isolates of this cosmopolitan pathogen may be found in the soil, air, equine feces and water, mainly in horse breeding farms. The disease occurs most commonly in foals up to 6 months of age and horses older than 1 year are rarely affected. When mature horses are affected, there is usually an accompanying immunodeficiency.1 There are sporadic reports of the disease in other species, including cattle, goats, pigs, dogs, cats, and humans. R. equi is an important cause of pneumonia in HIV-infected or otherwise seriously immunocompromised humans.5
This pathogen has a pathogenicity island, likely acquired through horizontal gene transfer from a bacterial source of unknown origin, critical for virulence of the bacterium for foals. The 26 coding sequences of this pathogenicity island include the virulence-associated protein (Vap family), which are exclusive to R. equi. There are 6 full-length vap genes (vap A, -C, -D, -E, -G, -H) and 3-truncated vap pseudogenes (vapF, -I and ?X). VapA is the vap gene with demonstrated role in virulence and encodes an immunodominant temperature inducible and surface-expressed protein. Vaps C, D, E, F, G, I and X appear to be dispensable. VapA is required for intracellular growth in macrophages and for the establishment of a persistent infection in severely immunodeficient mice. In macrophages, it aids in preventing maturation of the phagosome to the stage of fusion of R. equi containing vacuoles with lysosomes.3
Early signs of the usual chronic and progressive pulmonary form of the disease are fever (up to 41oC), lethargy, increased respiratory rate with bronchovesicular sounds over large airways and wheezing over small airways, cough and, sometimes, bilateral nasal discharge.1,3,4 As the lung lesions progress, foals present increases in respiratory rate and depth, and movement becomes increasingly distressful, leading to tachypnea, tachycardia and flared nostrils. Severe diarrhea might occur because of colonic mucosal invasion by the agent4 or secondarily to antibiotic treatment.1,3 Polysynovitis can occur in 40% or more of affected foals and result in no more than mild pain and decreased range of motion. Foals with septic polyarthritis are quite lame, as observed in this case. The most commonly affected sites include the tarsocrural, carpal, and fetlock joints.6
Intestinal lesions caused by R. equi are characterized by ulcerative pyogranulomatous enterocolitis. In this case, ulcers were evidenced grossly but were not present in the submitted slides, which had mainly multifocal pyogranulomas throughout the layers of the large colon. Intestinal infection seems to occur after the penetration of the specialized epithelium over Peyer's patches or intestinal lymphoid follicles by coughed up and swallowed bacteria. An initial neutrophilic response occurs leading to erosions of the epithelium (ulcers) with accumulation of macrophages and neutrophils in the lamina propria. The macrophages contain aggregates of R. equi but do not destroy them. Later necrosis of lymphoid follicles occurs with replacement by granulomatous inflammation, abscess formation and necrotic material. Infection then spreads to mesenteric lymph nodes with a similar result. Pyogranulomatous lymphangitis and mesenteric lymphadenitis characterize the chronic enteric disease.8
Classic form of the disease is pyogranulomatous bronchopneumonia, however, there are many extrapulmonary manifestations of R. equi infection. Abdominal manifestations of the disease include mesenteric lymphadenopathy, ulcerative pyogranulomatous enterotyphlocolitis, peritonitis, splenic granulomas or abscesses and large intra-abdominal abscesses. Nonseptic polysynovitis characterized by synovial effusion without lameness has been identified for a multitude of foals with R. equi pneumonia. Septic arthritis and osteomyelitis also have been reported, both with or without signs of pneumonia. Other less commonly reported EPD's include septic pleuritis, uveitis and hypopyon, pyogranulomatous hepatitis, intracranial abscesses, cellulitis and subcutaneous abscesses.6
Contributing Institution:
Veterinary School
Universidade Federal de Minas Gerais
www.vet.ufmg.br
JPC diagnosis:
1. Colon: Colitis, pyogranulomatous and necrotizing, chronic, multifocal to coalescing, severe, with pyogranulomatous lymphangitis and edema, and numerous intrahistiocytic coccobacilli.
2. Lymph node (per contributor): Lymphadenitis, pyogranulomatous and necrotizing, diffuse, severe, with numerous intrahistiocytic coccobacilli.
JPC comment:
The contributor provides a concise summary of Rhodococcus equi. First described in 1923 as the causative agent of "purulent bronchopneumonic disease in foals" by H. Magnusson in Sweden, it is currently one of 57 species within the Rhodococcus genus. Interestingly, only two species are currently considered pathogenic: R. equi, a significant pathogen in domestic species, and R. fascians, the causative agent of leafy gall in plants.9
As the contributor noted, the virulence associated proteins (vapA specifically) within the R. equi plasmid confer the ability to survive and replicate in macrophages. The full mechanism has not yet been discovered, but there is evidence that the bacterium shelters in an endosome within macrophages, called the R. equi-containing vacuole (RCV), the membrane of which has localized VapA.9
Separate from discussion of the vap plasmid of R. equi, the core genome also confers traits that allow for survival and pathogenicity in animals. Genes on the core genome help protect against desiccation and oxidative stress, and encode b-lactamases, aminoglycoside phosphotransferases, and multidrug efflux pumps. These genes augment this bacterium's ability to survive in the environment and resist antibiotic therapy and is highly conserved across strains.9
A recent description of six cases of R. equi in goats further characterized disease in this species. VapN was previously described in cattle samples, and all goats carried vapN positive strains, using PCR on all samples. An additional avirulent VapN-/VapA- sample was found in one animal. VapB is more common in pig isolates and was not found in these samples. The VapN plasmid is linear and is necessary for R. equi's ability to replicate within macrophages.7
Previous reports of R. equi in cats has failed to fully characterize the plasmid profile of isolates. A recent study of 200 cats in Brazil found approximately 3.5% had a single pathogen infection with R. equi, and approximately 4% had coinfection with R. equi and E. coli. Interestingly, none of the R. equi isolates contained the plasmids VapA, VapB, or VapN, and were considered avirulent. While this further characterizes R. equi in cats, further research is warranted to determine the public health impact this may represent for immunocompromised or immunosuppressed populations.2
In the moderator's experience, this case varied from the typical presentation of colitis arising from R. equi, with the mucosa largely intact in these sections. Fibrin thrombi were noted by some conference participants but were not evident in all sections. Approximately 50% of foals with R. equi pneumonia will also have pyogranulomatous enterotyphlocolitis, pyogranulomatous lymphadenitis, intra-abdominal abscesses, or peritonitis. The typical portal of entry is respiratory, but then expectorate is swallowed and enters the gastrointestinal tract.
References:
1. Cohen ND. Rhodococcus equi foal pneumonia. Vet Clin Equine. 2014;30(3):1-14.
2. de Paula CL, Silva ROS, Hernandes RT, et al. First Microbiological and Molecular Identification of Rhodococcus equi in Feces of Nondiarrheic Cats. BioMed Research International. 2019;4278598.
3. Guiguére S, Cohen ND, Chaffin MK, et al. Rhodococcus equi: clinical manifestations, virulence and immunity. J Vet Intern Med. 2011;25:1221-1230.
4. Prescott JF. Rhodococcus equi: an Animal and Human Pathogen. Clin Micro Rev. 1991;4(1):20-34.
5. Prescott JF, Meijer WG, Vazquez-Boland JA. Rhodococcus. In: Gyles CL, Prescott JF, Songer JG, Thoen CO, eds. Pathogenesis of Bacterial Infections in Animals. 4th ed. Ames, IA:Wyley-Blackwell; 2011:149-162.
6. Reuss SM. Chaffin MK. Cohen ND. Extrapulmonary disorders associated with Rhodoccocus equi infection in foals: 150 cases (1987-2007). J Am Vet Med Assoc. 2009. 235:855-863
7. Stranahan LW, Plumlee QD, Lawhon SD, Cohen ND, Bryan LK. Rhodococcus equi Infections in Goats: Characterization of Virulence Plasmids. Vet Path. 2018;55(2):273-276.
8. Uzal FA, Plattner BL, Hostetter JM. Alimentary System. In: Maxie MG, ed. Jubb, Kennedy and Palmer's Pathology of Domestic Animals. 6th ed. Vol2. St. Louis, MI: Elsevier; 2016:1-257.
9. Vazquez-Boland JA, Meijer WG. The pathogenic actinobacterium Rhodococcus equi: what's in a name? Molecular Microbiology. 2019;112(1):1-15.