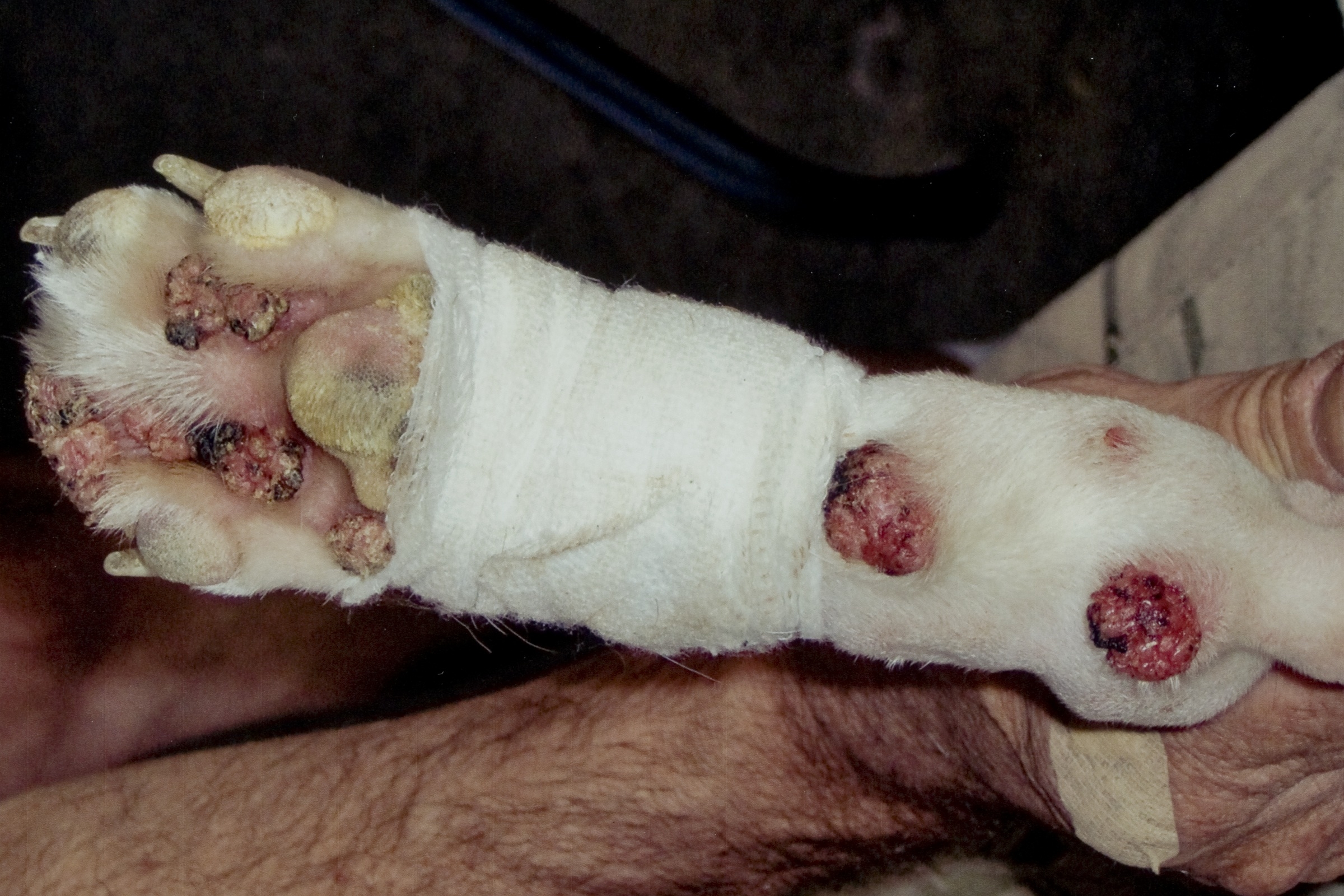
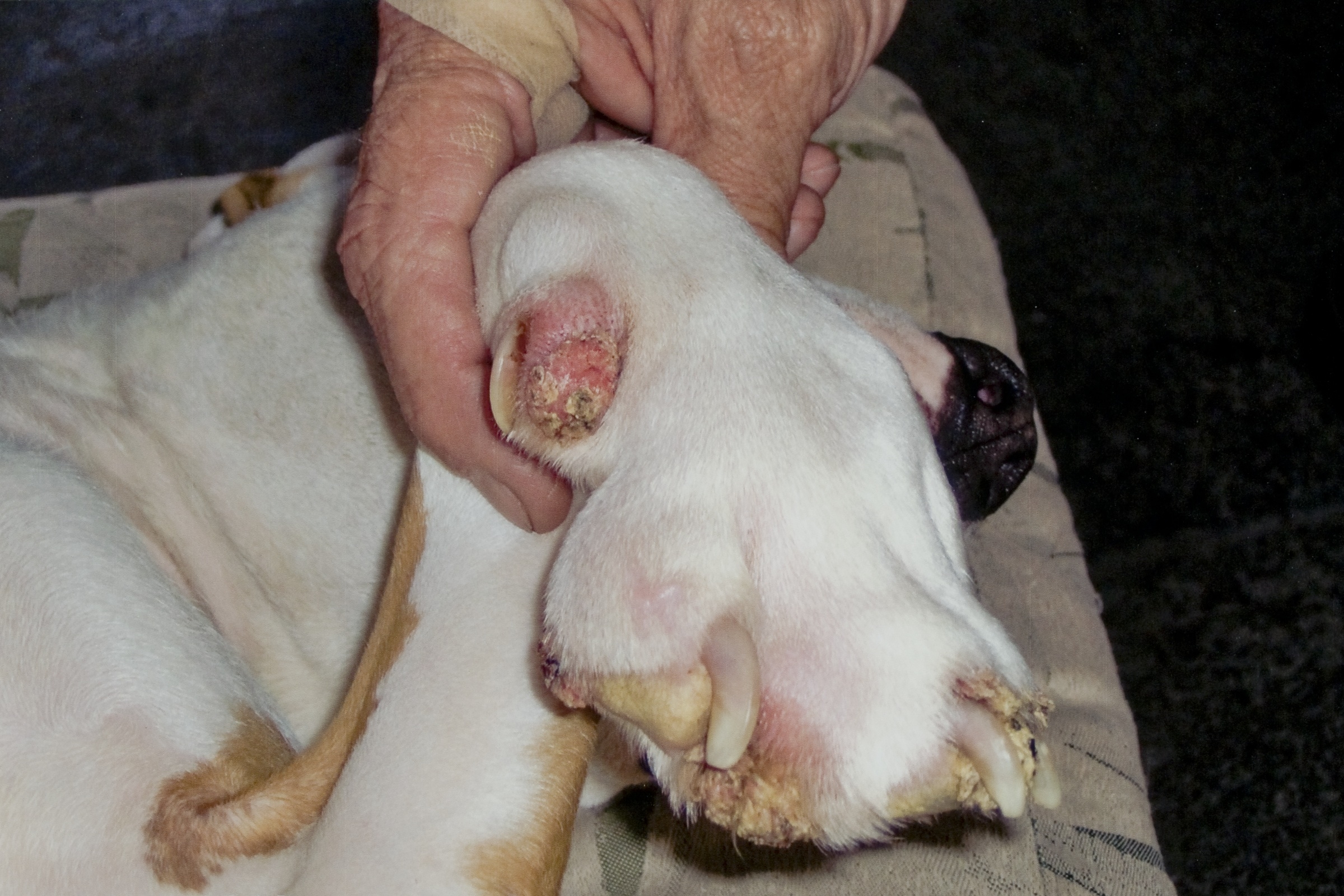
Signalment:
Gross Description:
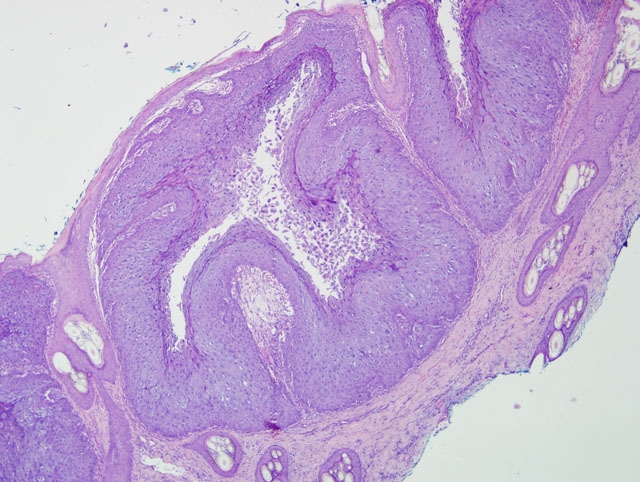
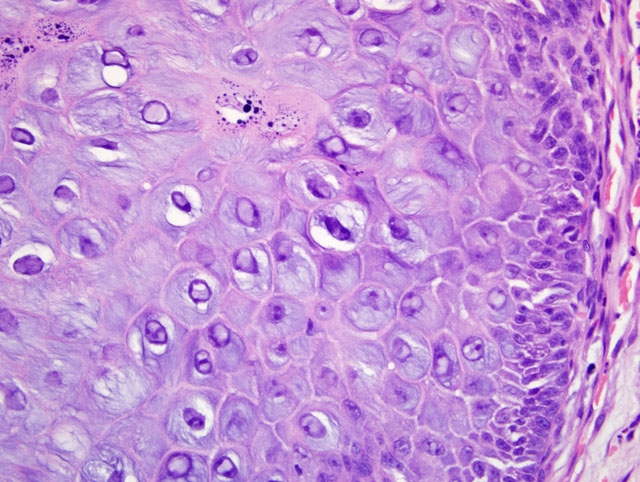
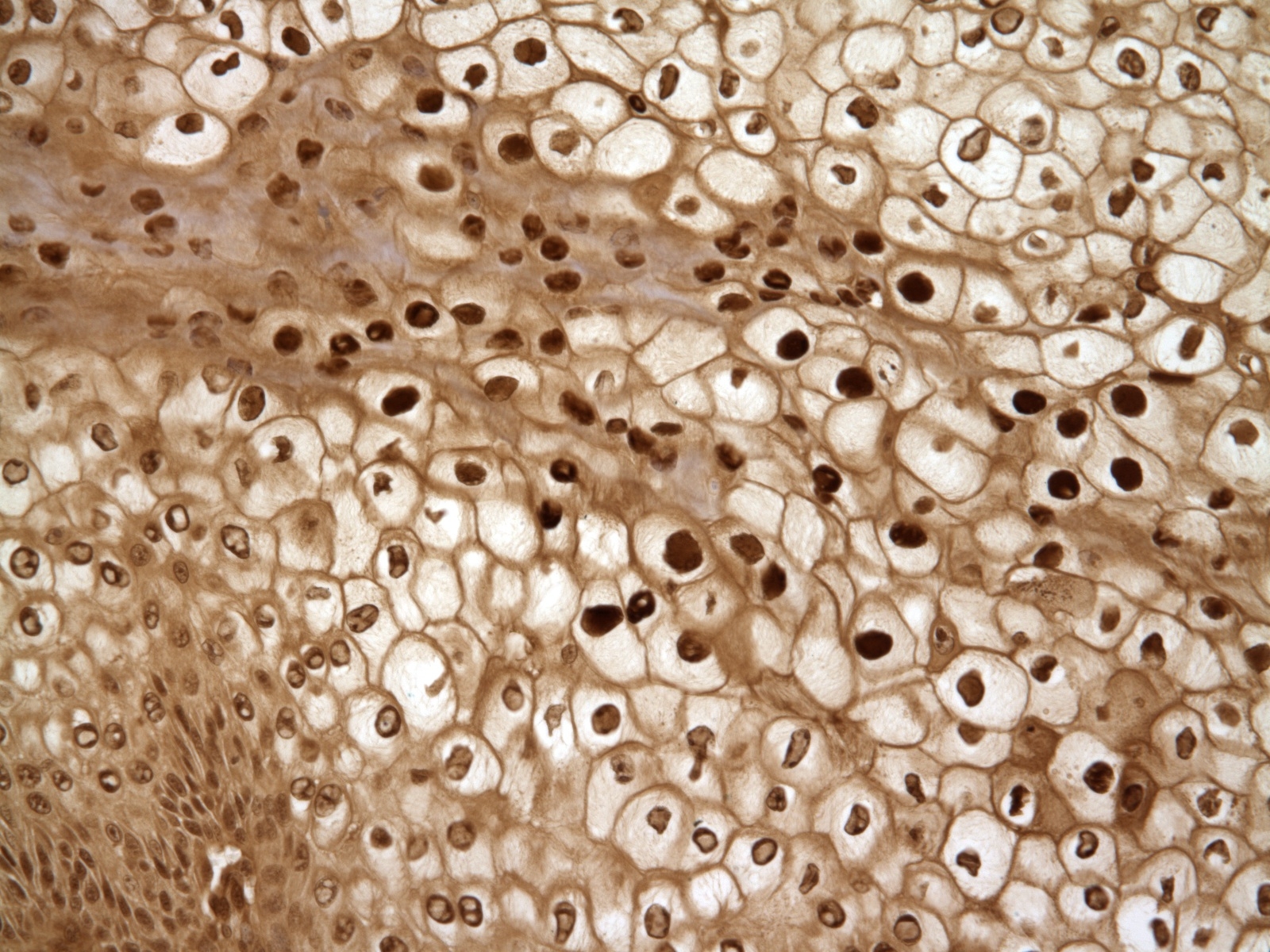
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
At least four different PVs are believed to infect dogs.(2) Classification of PVs is often based on the L1 gene, which encodes the viral capsid and packages viral DNA with L2, because it is the most conserved region of the PV genome.(1)1 Oral papillomatosis in dogs, characterized by multifocal cauliflower growths affecting the tongue, gingival, buccal mucosa, lips, and pharynx, is believed to be caused by the lambda papillomavirus COPV.(2) Dogs that clinically manifest oral papillomas are generally less than 3 years old, but papillomas can appear in immunosuppressed and older dogs. Papillomatosis in dogs is considered to be a self-limiting disease with spontaneous regression of tumors, so treatment is generally not recommended.(2) Occasionally, these tumors persist and undergo malignant transformation. PVs are associated with cutaneous neoplastic transformation in several species, including sarcoids in cats and horses, and squamous cell carcinoma in dogs, cats, rabbits, bandicoots, and rodents.(7)
It has been proposed that cutaneous papillomatosis is caused by a different PV. A novel, epidermotropic PV has been recently described, termed CfPV-2.(10) Unlike COPV, lesions associated with CfPV-2 appear to be restricted to the footpads, with more chronic lesions lasting greater than 6 months. Experimentally, CfPV-2 is unable to induce oral papillomas in immunocompetent dogs, and vaccination against COPV is not effective against CfPV-2.(10) In addition, chronic infection with CfPV-2 is associated with highly malignant squamous cell carcinoma with distal metastases, although the exact pathogenesis of CfPV-2-associated neoplastic transformation is not known. The link between different PVs and specific tumor manifestations is also unclear. Recently, genotyping of PVs associated with canine inverted papillomas discovered the presence of either CfPV-2, COPV, or unknown canine PVs, suggesting that more than one type of PV may cause inverted papillomas.(6)
Canine oral papillomatosis can provide a model for studying regression of warts in human PV-associated cervical papillomatosis, because the predictable nature of lesion regression in canine papillomas closely mimics regression in cervical warts. In canines, papilloma regression is associated with leukocyte influx, with an abundance of CD4+ and CD8+ lymphocytes.(8)
Differentials for this case include nail bed inverted squamous papilloma. These masses arise from nail bed epithelium, and histologically contain laminated, compact keratin within a hollow mass.(9) The nails are also grossly broken or missing; however, viral inclusions, lack of involvement of the nails, and lack of cyst formation rule out nail bed inverted papilloma. In this case, the papilloma likely originated from the skin adjacent to the nail bed, consistent with previous reports.(2) In the absence of viral inclusions, other differentials include cutaneous squamous papilloma of non-viral origin.
JPC Diagnosis:
Conference Comment:
The precise role that papillomaviruses play in the development of cutaneous neoplasia in animals is not entirely understood. It has been proposed that ultraviolet light and papillomaviruses may act as co-carcinogens. Ultraviolet light causes damage to nuclear DNA, which increases the likelihood of oncogenic transformation.(3) Simultaneously, papillomavirus infection promotes epithelial proliferation.(4)
Many important papilloma viruses of animals exist and are beyond the scope of this discussion; those interested are invited to consult the references.(3,7) The chart below summarizes several papilloma viruses in animal species:
| Species | Virus | Tissue(s) affected | Disease(s) |
| Ox | Bovine papilloma virus (BPV) 1, 2 & 5 | Udder, teats, head, neck, shoulders, omasum, vagina, vulva, penis and anus; BHV-2 also affects the urinary bladder | Teat frond warts; cutaneous warts; rice grain fibropapillomas; transmissible fibropapilloma in bulls |
| Ox | BPV-3 | Skin | Papillomas of the skin |
| Ox | BPV-4 | Alimentary tract e.g. mouth, pharynx and upper alimentary tract | Bovine alimentary papillomata; associated with squamous cell carcinoma and squamous papilloma of the alimentary tract and urinary bladder |
| Equine | BPV-1 & -2 | Skin | Sarcoid |
| Sheep | OvPV-1 | Skin | Fibropapillomas |
| Feline | FdPV-1 & -2 | Skin | Viral plaque progressing to Bowenoid carcinoma in-situ; FdPV-2 linked to squamous cell carcinoma |
| Feline | FdPV-2 | Skin | Squamous cell carcinoma (SCC) |
| Canine | CfPV-3 & -4 | Skin; mucous membranes of the eye, skin and genitals | Papillomas; viral plaque progressing to Bowenoid carcinoma in-situ and SCC |
| Canine | CfPV-2 | Skin | Inverted papilloma; SCC |
| Rabbit | CRPV | Tongue; skin | Lingual papillomas; SCC |
| Western barred Bandicoot | BPCV1 | Skin | SCC |
References:
2. Debey BM, Bagladi-Swanson M, Kapil S, Oehme FW. Digital papillomatosis in a confined Beagle. J Vet Diagn Invest. 2001;13:346-348.
3. Ginn PE, Mansell EKL, Rakich PM: Skin and appendages. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. 5th ed., vol. 1. Philadelphia, PA: Elsevier Ltd; 2007:647-648
4. Goldschmidt MH, Dunstan RW, Stannard AA, von Tscharne C, Walder EJ, Yager JA. Histological classification of epithelial and melanocytic tumors of the skin of domestic animals, 2nd series, vol. III. Washington, D.C.: Armed Forces Institute of Pathology; 1998:19-20.
5. Kusewitt DF, Rush LJ. Neoplasia and tumor biology. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 4th ed. St. Louis, MO: Elsevier; 2007:265-267.
6. Lange CE, Tobler K, Brandes K, et al. Canine inverted papillomas associated with DNA of four different papillomaviruses. Vet Dermatol. 2009;21:287-291.
7. Munday JS, Kiupel M. Papillomavirus-associated cutaneous neoplasia in mammals. Vet Pathol. 2010;47:254-264.
8. Nicholls PK, Moore PF, Anderson DM, et al. Regression of canine oral papillomas is associated with infiltration of CD4+ and CD8+ lymphocytes. Virology. 2001;283:31-39.
9. Plattner BL, Hostetter JM. Cutaneous viral papilloma with local extension and subungual cyst formation in a dog. J Vet Diagn Invest. 2009;21:551-554.
10.Yuan H, Ghim S, Newsome J, et al. An epidermotropic canine papillomavirus with malignant potential contains an E5 gene and establishes a unique genus. Virology. 2007;359:28-36.
11.Yhee JY, Kwon BJ, Kim JH, et al. Characterization of canine oral papillomavirus by histopathological and genetic analysis in Korea. J Vet Sci. 2010;11:21-25.