CASE III: 20-23 (JPC 4118594).
Signalment:
4-month-old, male, F344-Il2rg/Rag2em1Iexas rat (Rattus norvegicus domestica)
History:
A cohort of 9 adult rats were received in order to expand a breeding colony for transplant research. Within 2 months, all animals aside from 1 female had either died or were euthanized in moribund condition. Clinical symptoms in rats that had them included hunching, weight loss, ataxia, and swollen limbs. Other animals were found dead with no previous clinical symptoms.
Gross Pathology:
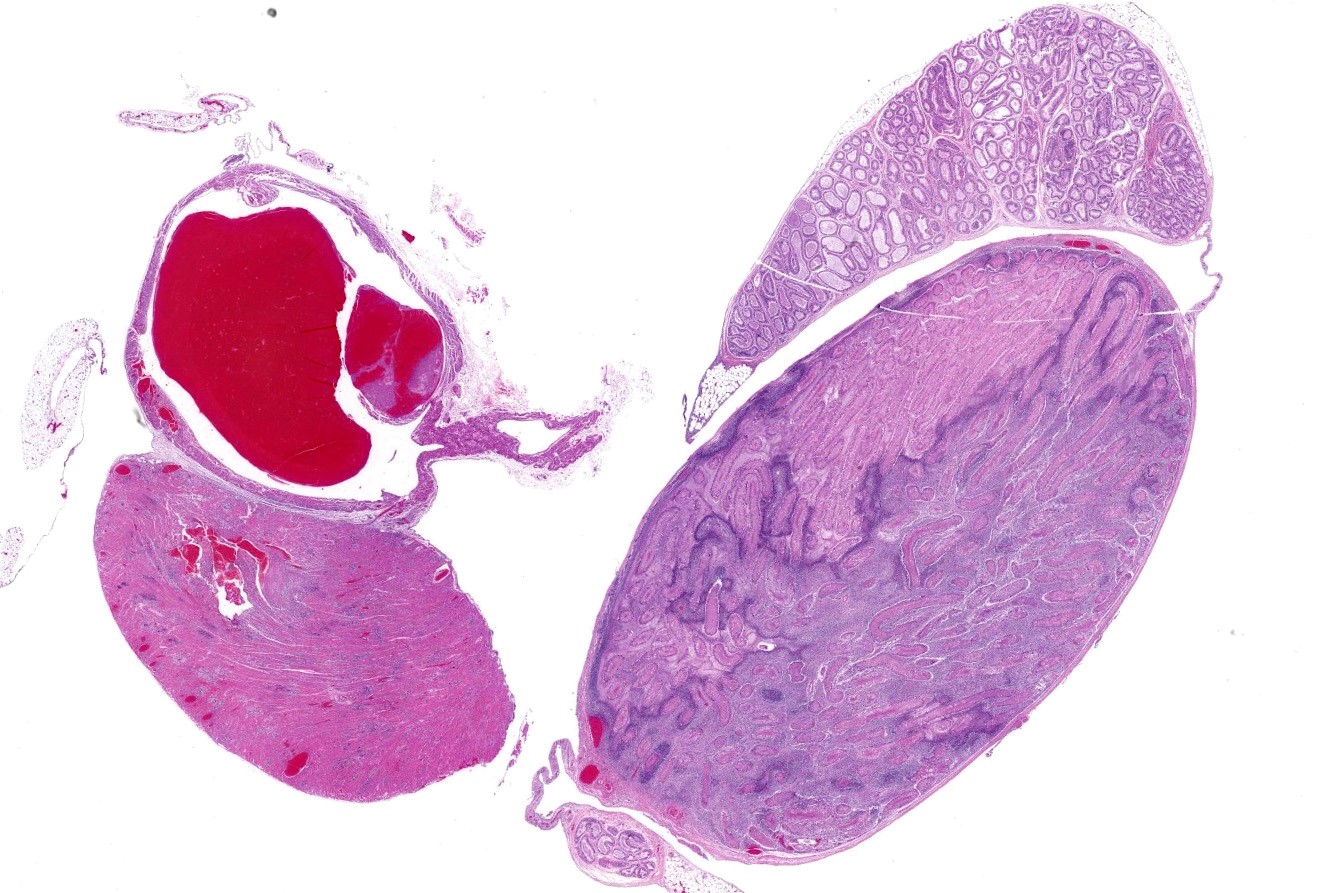
Some animals had no gross lesions while others had white foci in various organs, most often the heart, kidneys, and livers. This animal also had enlarged, white-tan testes.
Laboratory results:
Streptococcus suis was isolated from the blood via bacterial culture/sensitivity at necropsy.
Microscopic Description:
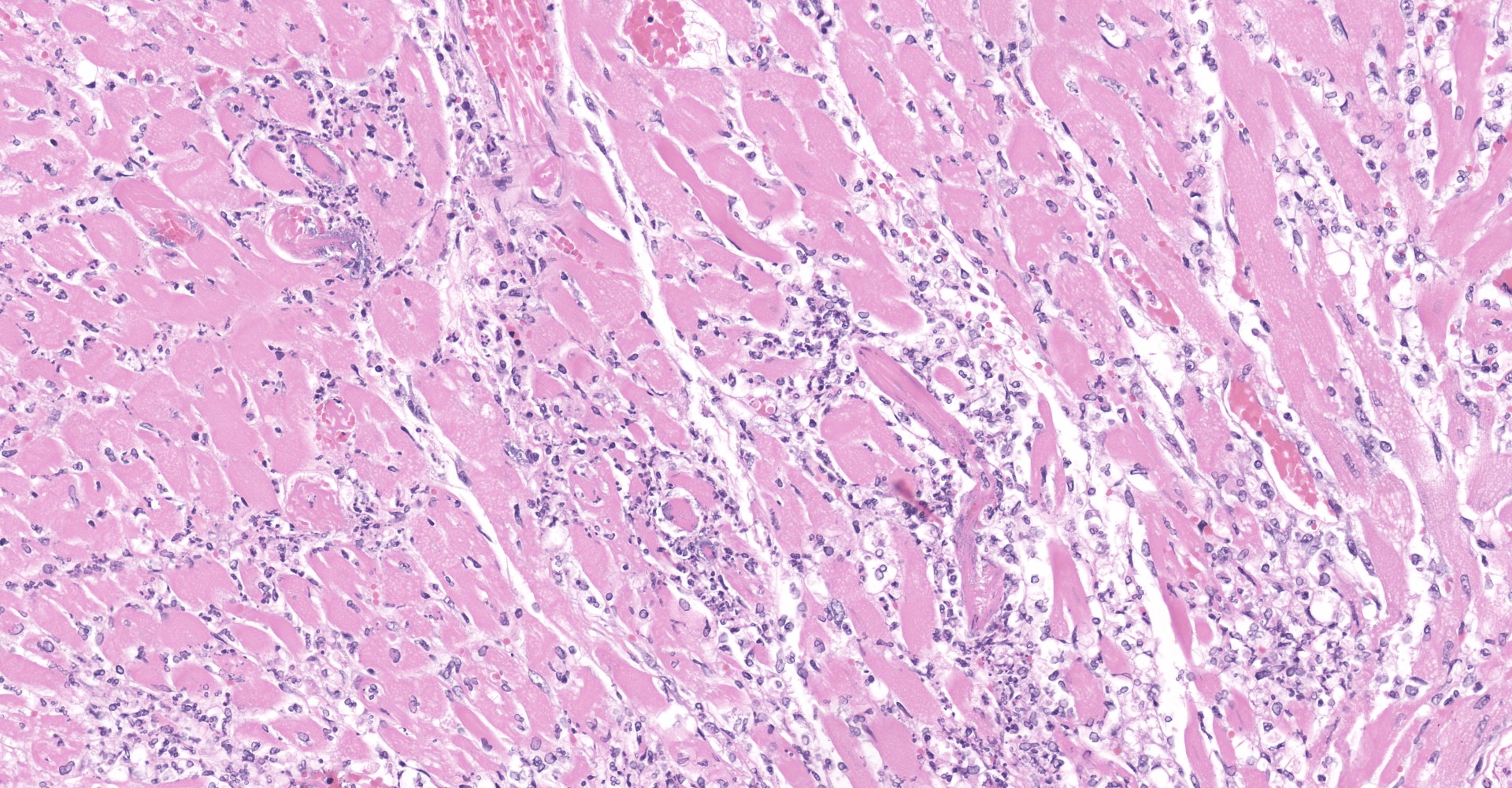
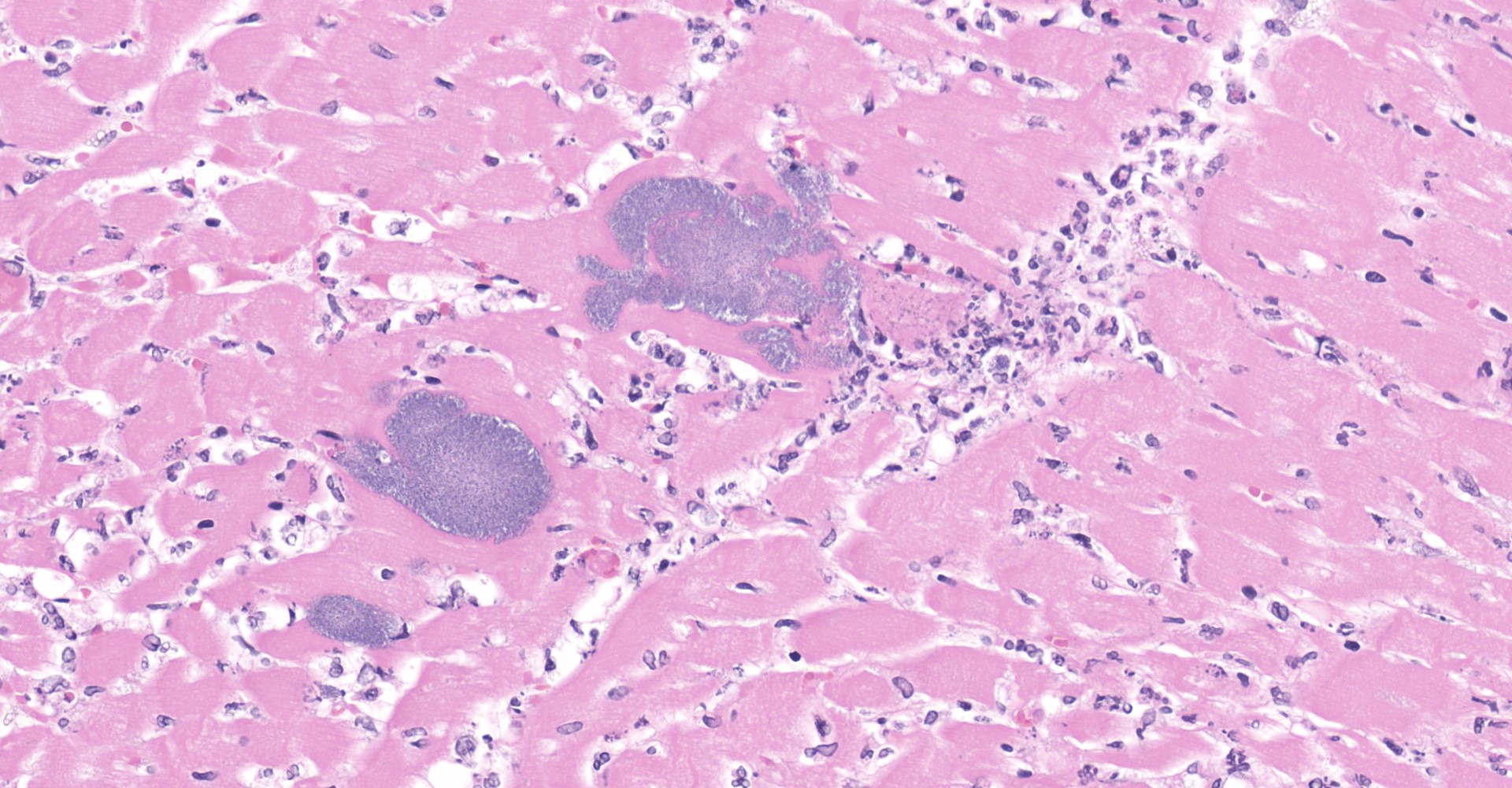
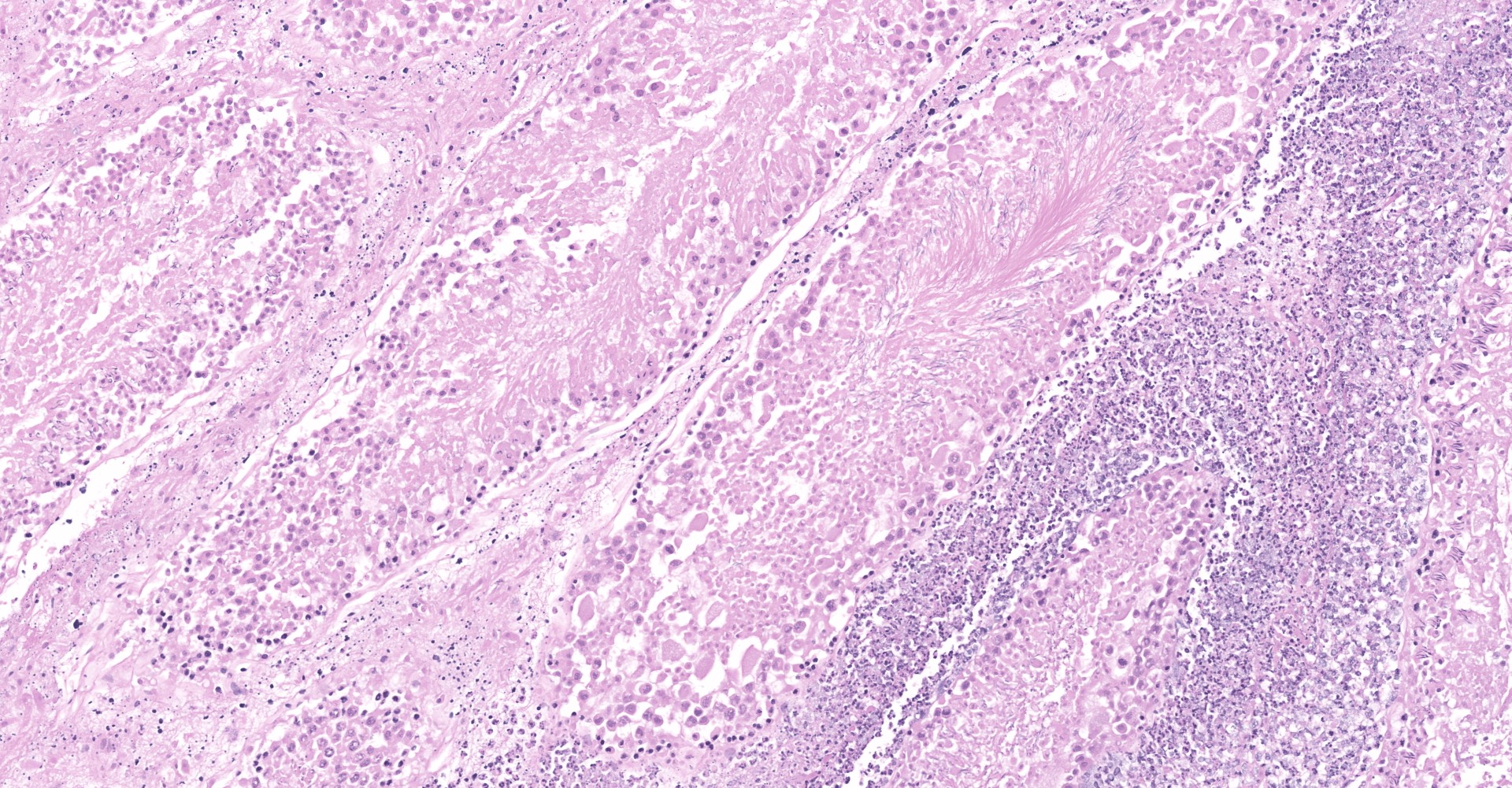
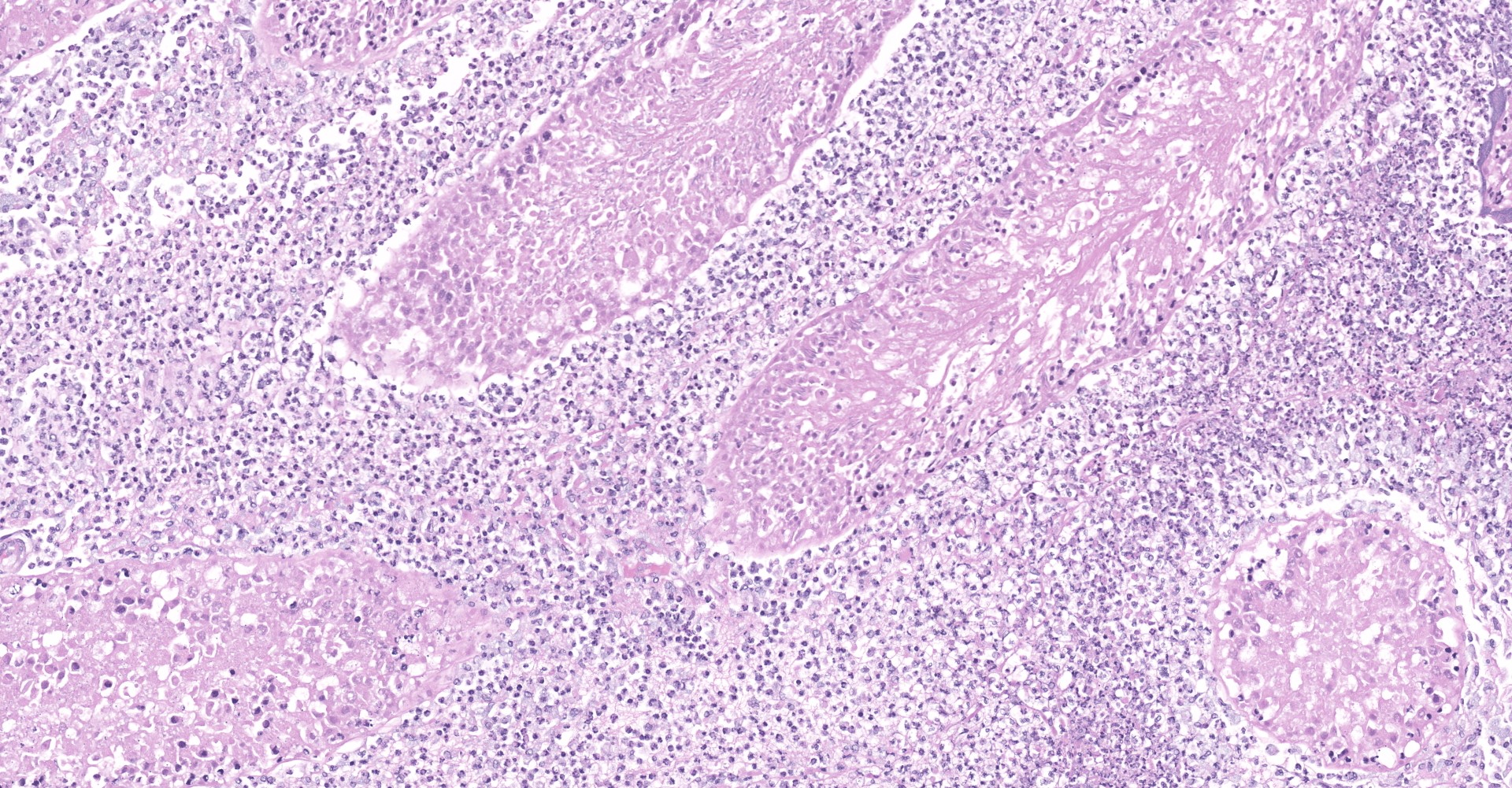
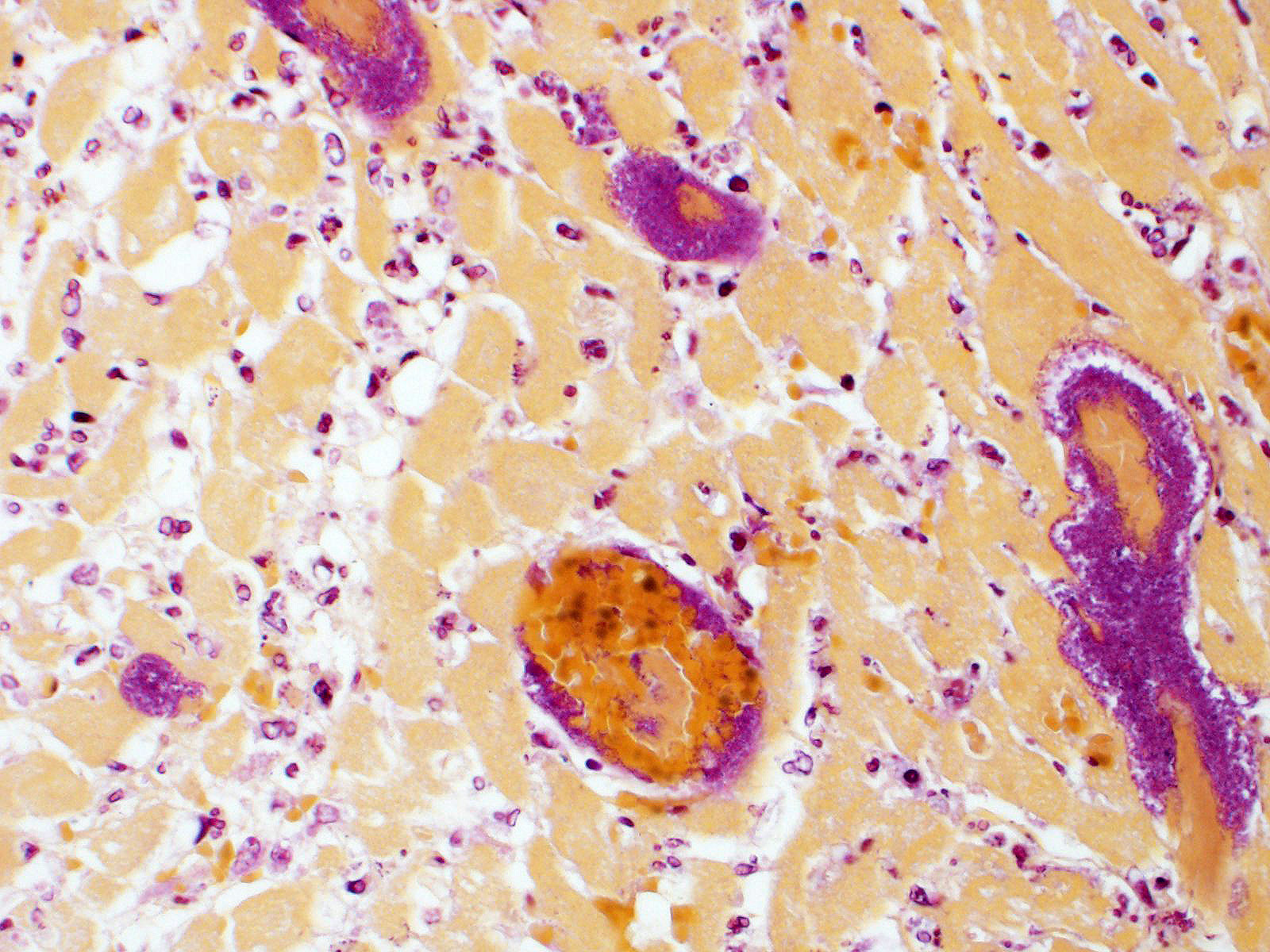
Heart: Throughout the myocardium, there are multifocal to coalescing aggregates of neutrophils and fibrin which separate, surround, and replace cardiomyocytes. Remaining cardiomyocytes in these areas either swollen with vacuolated sarcoplasm (degenerate), or shrunken with hypereosinophilic sarcoplasm, loss of cross striations, and pyknotic nuclei (necrotic). Multifocally, blood vessels contain adherent fibrin thrombi that contain enmeshed neutrophils and colonies of bacterial cocci.
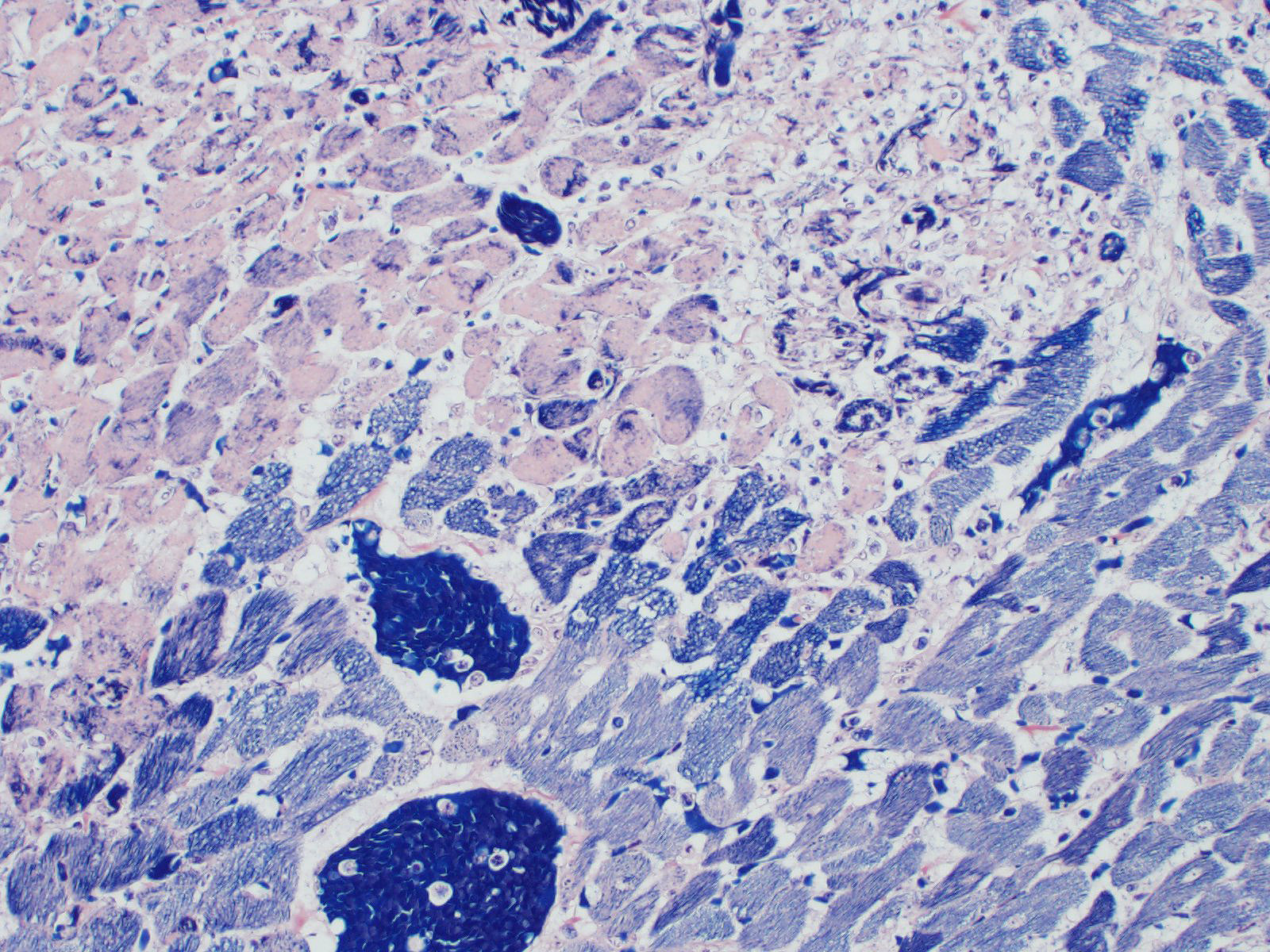
Testis: Diffusely within the interstitium, separating and surrounding seminiferous tubules are aggregates of neutrophils (often degenerate), fibrin, macrophages, and colonies of bacterial cocci. Intravascular fibrin thrombi and necrosis of blood vessel walls are frequently observed as are areas of lytic and/or coagulative necrosis. Seminiferous tubules are degenerative to completely necrotic (degree varies based on slide) with Sertoli cell vacuolation, spermatocyte necrosis, giant spermatids, and disorganization/loss of germ cells.
Contributor's Morphologic Diagnosis:
1. Heart:
Myocarditis, suppurative, severe, multifocal to coalescing, with vascular
thrombi and intralesional bacterial cocci
2. Testis: Orchitis, necrosuppurative, severe, diffuse with vascular thrombi and intralesional bacterial cocci
Contributor's Comment:
The F344-Il2rg/Rag2 em1Iexas rat strain shows severe combined immunodeficiency caused by 5-bp deletion in Il2rg gene on the X chromosome and 1-bp insertion in Rag2 gene on chromosome 3.4 Like their mouse counterparts, these rats have high immunocompromised symptoms due to the simultaneous absence of mature T-cells, B-cells, and NK cells as well as defective macrophage activity and reduced dendritic cell function.5 This model is ideal for studies involving implantation of human tissue; however, they are also extremely susceptible to secondary infections, particularly at the time of transport. Necrosuppurative lesions were also found in other organs including kidneys, meninges, joints (one animal), and liver. The testicular lesions were only noted in a single animal, and both testes were affected.
Streptococcus suis causes well-defined streptococcal septicemia in swine. The organism is carried in the palatine tonsils of pigs, and infection is likely spread via the respiratory route. Although outbreaks may occur, sporadic and isolated disease is more common. Lesions most frequently include purulent meningitis and polyserositis. Additional lesions that can be seen include purulent arthritis, fibrinopurulent pericarditis, endocarditis, or hemorrhagic, necrotizing myocarditis. Serotype 2 is the most commonly isolated serotype isolated from pigs and humans and is the most pathogenic serotype.1
S. suis is a significant human pathogen, particularly in southeast Asia, posing a challenge to public health.1,2 Human disease most frequently can include meningitis, septicemia, and pneumonia with arthritis, endocarditis, streptococcal toxic shock-like syndrome (STSLS) occurring less commonly.2
An interesting feature of this case is the severity of the lesion in the testis. This is not a commonly described feature of S. suis in any species. There is a case report in a human from Thailand experiencing acute pyogenic arthritis with orchitis also described, though details of the testicular lesions are not detailed.4
Contributing Institution:
Division of Laboratory Animal Resources
University of Pittsburgh
S1040 Thomas E. Starzl Biomedical Science Tower
200 Lothrop Street
Pittsburgh, PA 15261
http://www.dlar.pitt.edu/
JPC Diagnosis:
1. Heart: Myocarditis, necrotizing and suppurative, multifocal to coalescing, marked, with vasculitis and thrombosis, mural endocarditis, and large colonies of bacterial cocci.
2. Testis: Orchitis, necrotizing, diffuse, severe (infarct), with necrotizing arteritis and large colonies of bacterial cocci.
JPC Comment:
The contributor provides a concise overview of the spectrum of lesions in swine and humans caused by Streptococcus suis in addition to the unique circumstances likely contributing to the lesions observed in this case.
S. suis is a gram-positive facultative anaerobe that is spherical to oval in shape and exists in pairs and short chains. When cultured on blood agar, a zone of green discoloration is formed around colonies (α-hemolysis).2
S. suis can be cultured from blood or CSF samples obtained from septicemic patients and/or those with meningitis via standard microbiological techniques; however, it is often misidentified or the infection goes undiagnosed. Based on one author's experience, S. suis is commonly misidentified and reported as Streptococcus species α-hemolyic or viridans streptococci, Enterococcus faecalis, Aerococcus viridans, and S. pneumonia.6
35 S. suis serotypes (1 to 34 and 1/2) were initially identified based on the antigenic composition of the bacterial capsule during early classification.3 However, subsequent studies have demonstrated serotypes 32 and 34 belong to Streptococcus orisratti and analysis of 16S ribosomal RNA of serotypes 20, 22, and 26 revealed these serotypes are also genetically different, resulting in a new species coined Streptococcus parasuis. Therefore, S. suis is currently composed of 29 serotypes, with serotype 2 strains recognized as the most virulent and widely studied.3
In regard to zoonosis, S. suis is a worldwide threat due to its distribution, although the majority of reported human infections originate in Southeast Asia.6 Studies in Vietnam and China have found occupational or household exposure occurring during the slaughtering and trimming process of pig carcasses and the handling and consumption of pork-derived products are significant risk factors for zoonotic transmission. In addition, farmers in these countries were found to be at increased risk due to the common practices of sharing accommodations with pigs and the slaughtering and consumption of diseased animals at home.6
A systematic survey of bacterial meningitis in Vietnamese adults recently suggested that S. suis serotype 2 is the most common pathogen associated with human bacterial meningitis within that population. Although mortality was relatively low (2.6%), hearing loss was a common complication (66.4%).2
References:
1. Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol. 2. 6th ed. London, UK: Saunders Elsevier; 2016. Volume 1:150-151.
2. Feng Y, Zhang H, Wu Z, et al. Streptococcus suis infection: an emerging/reemerging challenge of bacterial infectious diseases?. Virulence. 2014;5(4):477-497.
3. Haas B, Grenier D. Understanding the virulence of Streptococcus suis: A veterinary, medical, and economic challenge. Med Mal Infect. 2018;48(3):159-166.
4. Suksuphew S, Chainarongsiriporn R, Ganokroj P. Acute Oligoarthritis with Streptococcus Suis Infection with Septicemia: A Case Report. Arc Cas Rep CMed 2018:3(3): 159.
5. The Institute of Experimental Animal Sciences (IEXAS), Graduate school of Medicine, Osaka University, SCID Rat by NBRP-Rat, viewed 24 June 2020, http://www2.med.osaka-u.ac.jp/gerdc/srpo/index_en.html.
6. Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis. 2009;48(5):617-625.
7. Zhao Y, Liu P, Xin Z, et al. Biological Characteristics of Severe Combined Immunodeficient Mice Produced by CRISPR/Cas9-Mediated Rag2 and IL2rg Mutation. Front Genet. 2019;10:401.