CASE II: 1235813-015 (JPC 4167863)
Signalment:
Juvenile male intact Cynomolgus macaque (Macaca fascicularis)
History:
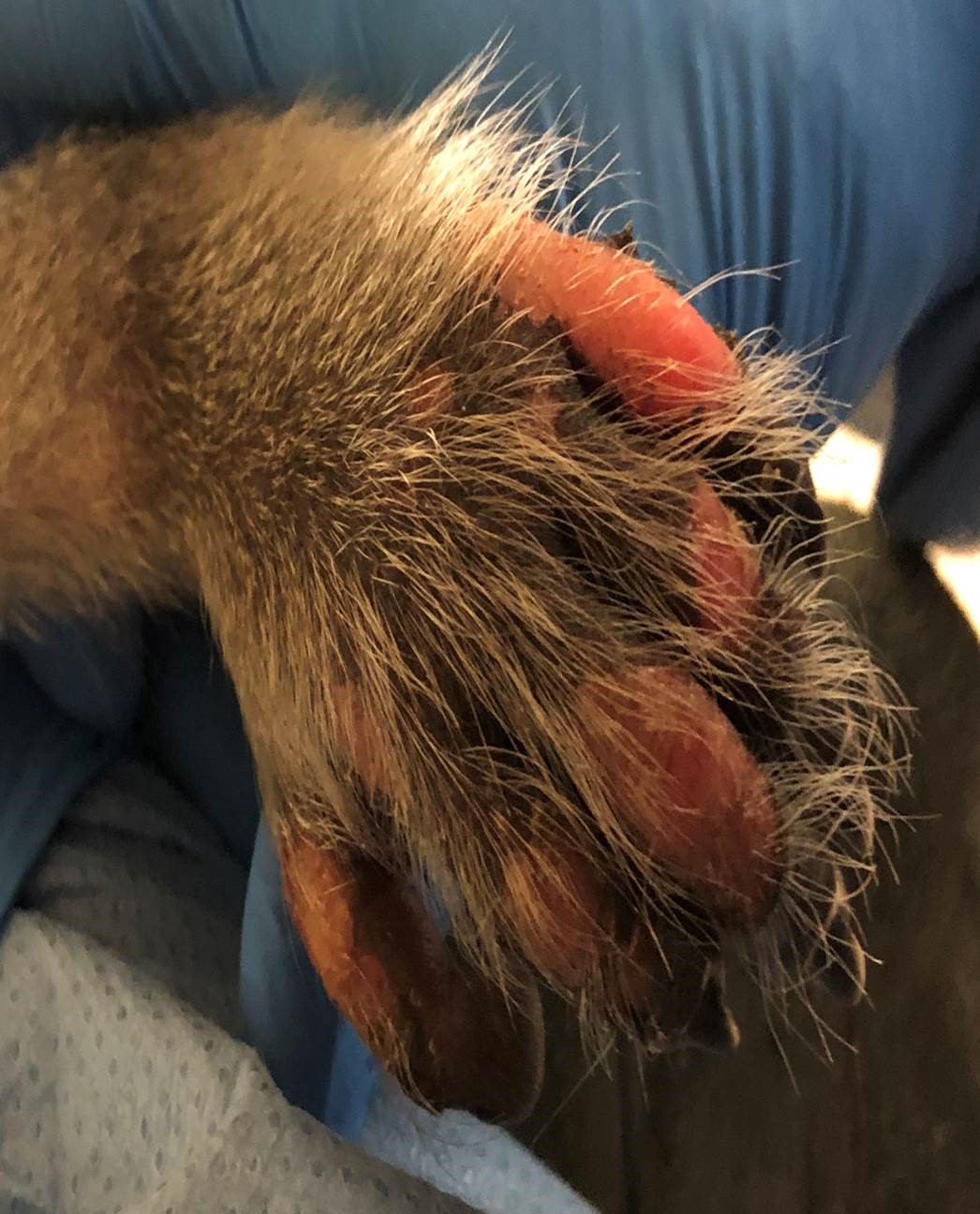
A laboratory primate that was in a control group was dosed intrathecally with radiolabeled imaging medium. Within an hour, the monkey developed vesicular lesions and skin sloughing on the tail, toes, and dorsal aspects of the feet.
Gross Pathology:
The skin of both hind feet was thickened, and there were multifocal abrasions and scabs on the hindfeet and tail.
Laboratory Results:
Haired skin, tail and glabrous skin, footpad: Dermatitis and folliculitis, necrotizing and vesicular, multifocal to coalescing, marked, with keratinocyte apoptosis.
Microscopic Description:
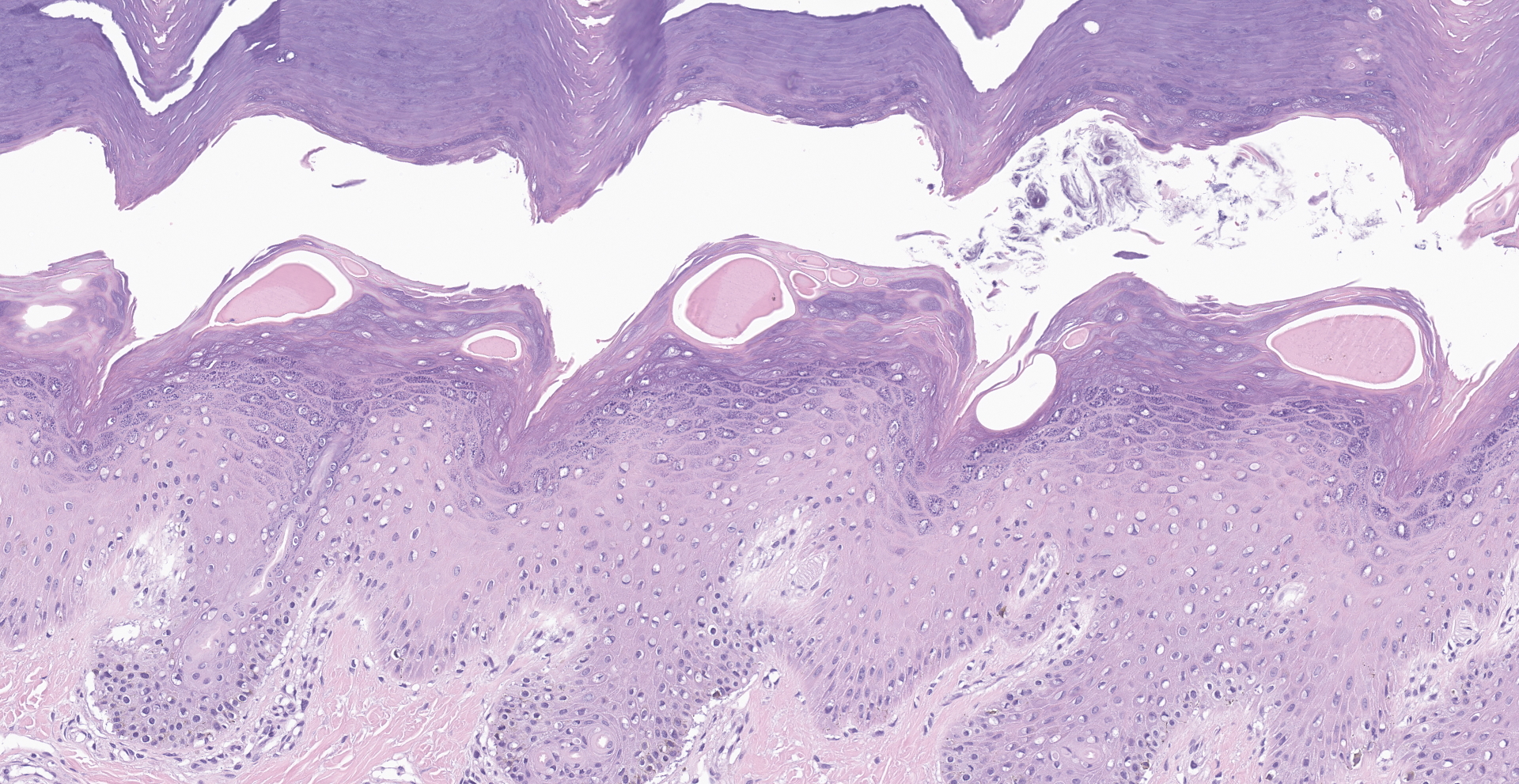
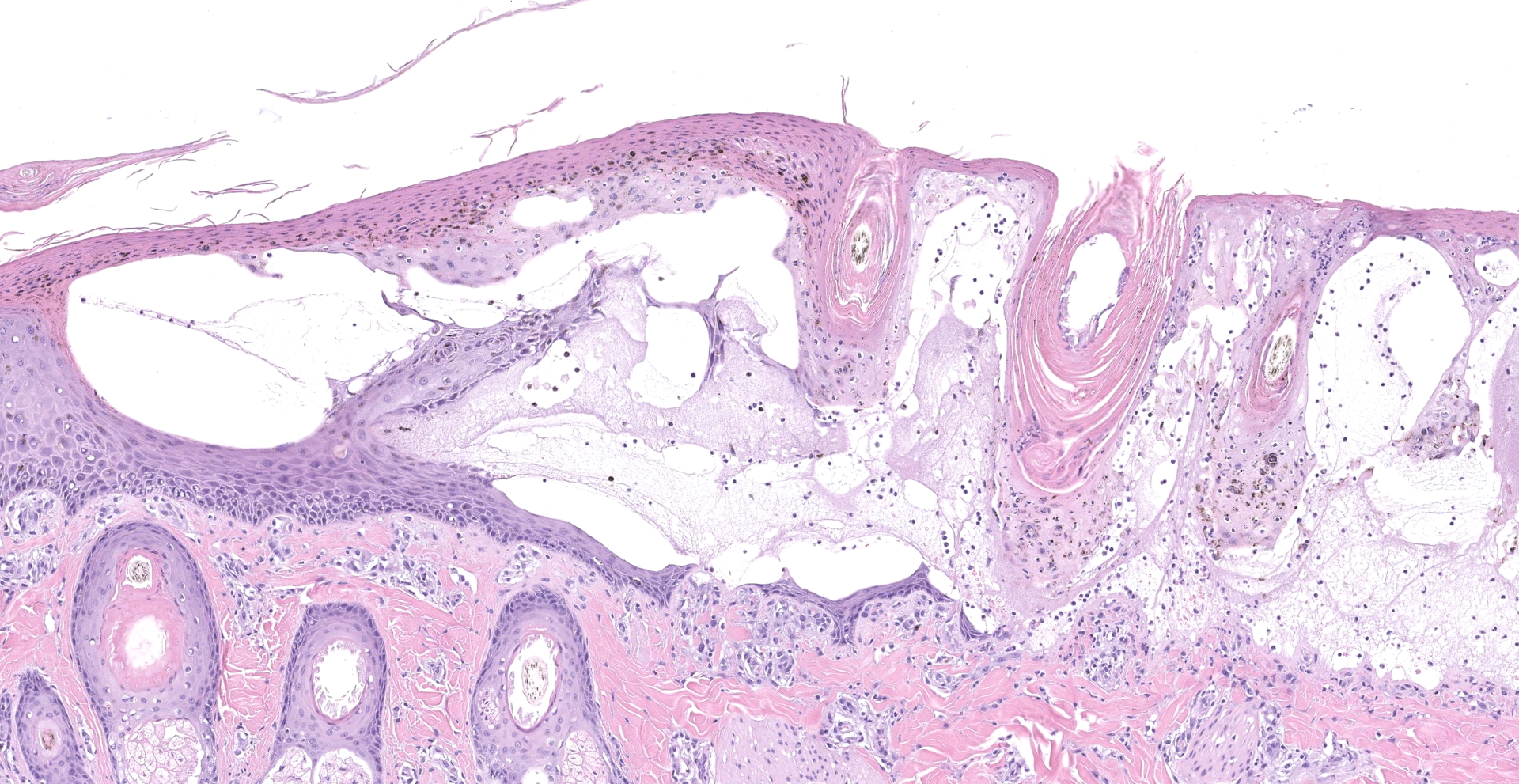
Within the haired skin of the tail and foot, there are multifocal intraepidermal vesicles and bullae characterized by variably sized open spaces within the stratum spinosum filled with loosely organized fibrillar material (fibrin), hemorrhage, small numbers of neutrophils, and few scattered melanin-laden macrophages. These vesicles are occasionally subcorneal, and focally they coalesce resulting in an extensive region of dermal-epidermal separation characterized by the stratum basale separating from the underlying superficial dermis. In a section specially stained with Periodic Acid-Schiff, the highlighted basement membrane is retained along the dermis in this area. The resulting space contains a large amount of fibrin, fluid, macrophages, and neutrophils. Within the separated epidermis and underlying follicular epithelium, keratinocytes of the stratum basale and stratum spinosum are swollen with pale eosinophilic to clear cytoplasm with multifocal large clear vacuoles (ballooning degeneration). Throughout all layers in the epidermis and follicular epithelium, there are multifocal shrunken and hypereosinophilic keratinocytes (apoptosis). Rarely, there are few lymphocytes adjacent to these apoptotic cells. Multifocally, there are small areas of lifted epidermis that have loss of cellular detail, hypereosinophilic cytoplasm, and pyknotic nuclei (necrosis). Underlying the lifted epidermis, the superficial dermal collagen is mildly smudged, and superficial small vessels are lined by plump reactive endothelium and surrounded by small numbers of neutrophils and a small amount of hemorrhage and edema. Away from larger bullae and vesicles, the stratum basale and stratum spinosum are mildly to moderately vacuolated (spongiosis). Occasionally, there
are free hair shafts within the bullae that are surrounded by moderate numbers of neutrophils and macrophages. There are occasional colonies of bacterial cocci in these areas and within rare follicles.
Contributor's Morphologic Diagnoses: Haired skin, tail and foot: Multifocal to coalescing subepidermal and intraepidermal vesicles and bullae, multifocal keratinocyte apoptosis and necrosis, locally extensive ballooning degeneration and spongiosis, and mild superficial neutrophilic dermatitis.
Contributor's Comment:
Cutaneous adverse drug reactions can occur through several mechanisms and have been reported in macaques.1,3,6,8,10 First, there could be direct physical or chemical insult to the skin. There could also be indirect toxicity mediated by immune reaction. This occurs with immediate or delayed reactions. The most common cutaneous adverse drug reactions are irritant contact dermatitis and allergic contact dermatitis, which may both be characterized by erythematous macules or vesicles with underlying intercellular edema and superficial dermatitis.8 Irritant contact dermatitis occurs rapidly after the insult, and acute contact dermatitis is a delayed type IV hypersensitivity reaction. Other possible cutaneous adverse drug reactions include rash with eosinophilia and systemic symptoms (DRESS), generalized exanthematic pustulosis, erythema multiforme (EM), fixed drug eruption, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN).8 It is important to differentiate these types of lesions from other potential causes of these lesions in macaques such as measles, simian varicella virus, Simian immunodeficiency virus, and pox virus.
In this case, multiple primates presented with similar lesions on the feet and tail after being dosed with a radiolabeled imaging compound. It is important to note that both control and test-article treated animals were affected in this case, and the only common exposure was the radiolabeled substance used for imaging purposes. The monkeys were housed in the same room; however, they were located far apart from each other within the room. There were also other monkeys on the study that had no skin lesions. Animals affected were dosed intrathecally, subcutaneously, or intravenously. Interestingly, after switching manufacturers of the isotope, no additional lesions occurred in any other monkeys.
Two monkeys with lesions recovered; however, the others were euthanized for welfare reasons. An inciting cause of these lesions and a precise syndrome were not able to be confirmed; however, this most likely represents an adverse cutaneous reaction to the radiolabeled imaging medium. There was a higher level of the radioactive substance present within the vesicles as compared to the serum of the animals. This accumulation within the vesicles may have exacerbated the lesions. The bacteria present within the lesions are considered to be a complicating factor and not the inciting cause.
Several differential diagnoses were considered in this case based on the clinical presentation, presence of vesicles, bullae, apoptotic cells, and neutrophilic inflammation. Viral etiologies were excluded based on the clinical presentation and absence of characteristic lesions such as inclusions and syncytia.2,8 Based on the gross and histologic lesions, bullous and necrotizing epidermal diseases were considered as the primary differentials.
The presence of apoptotic cells in all cell layers raised concern for a diagnosis of EM. Characteristically in this disease, there will be single or multiple keratinocytes in a group that are hypereosinophilic, shrunken, and have pyknotic nuclei. These will be present at all levels of the epidermis and extend into hair follicles. Apoptosis is induced by lymphocytes, and these lymphocytes will surround the apoptotic cells (satellitosis).4,11 The presence of lymphocytes was not a prominent feature observed in this case. There were rarely single lymphocytes in the area of the apoptotic cells. Therefore, while remaining a differential, this condition was considered less likely. In cases of EM there may also be secondary ulceration, crusting, and neutrophilic inflammation. Parakeratosis may also be present in some cases of EM.4,11
TEN has been reported to occur with adverse drug reaction, and has been reported in macaques.1,3 The large areas of apoptosis in this case also histologically resemble toxic epidermal necrolysis; however, the clinical picture does not fit with this condition. In TEN greater than 30% of the skin should be affected, and TEN is a life-threatening condition. In TEN there is also bullous detachment of the epithelium from the dermis, which was observed in some areas in this case. Ultimately this will lead to ulceration. While there may be some lymphocytes present, there are usually present in smaller numbers than in EM. There is also usually little inflammation present.4,11 It is debated in both the human and veterinary literature whether TEN represents a fulminant form of EM or whether they are distinct disease entities. The clinical picture and progression of disease will aid in distinguishing the two diseases.11 Additionally, Stevens-Johnson syndrome (SJS) is also considered by some to be related to TEN and EM, and can have similar histologic findings. Due to the clinical picture (less than 30% of skin affected), frequent presence of neutrophilic inflammation, and vesicles within the stratum corneum, TEN was considered unlikely, even though there was an area in the tail with detachment of the epidermis from the dermis and presence of apoptotic cells with little inflammation.
Bullous pemphigoid-like disease has also been reported in macaques.6 This is another disease that can cause blister lesions. In this condition there is development of autoantibody to the bullous pemphigoid antigen, a transmembrane glycoprotein in basal keratinocytes.4 The dermo-epidermal junction is then separated by proteinases released by granulocytes.6 The subepidermal separation differentiates this condition from pemphigus vulgaris which may have a similar gross appearance. This condition presents with erythematous macules and vesicles that progress to bullae and ulceration. Typically, both skin and mucous membranes will be affected. Histologically, there will be clean separation between the dermis and epidermis forming a cleft. Neutrophils, eosinophils, and fibrin may be present along the surface of the separated dermis.4,6 Since some vesicles were intraepidermal, this differential was considered less likely.
Pemphigus vulgaris (PV) is a severe vesiculobullous and ulcerative disease that can occur as a drug reaction.4 This disease targets the autoantigen Desmoglein 3 in the mucous membranes, which is expressed in suprabasilar keratinocytes.4 Autoantibodies binding to this autoantigen result in acantholytic cells.4 Cases of PV can present with symmetric transient vesicles, bullae, and ulcers/erosions on the mucous membranes and around areas with friction such as axillary, inguinal, and pressure point areas. Histologically cases will have a suprabasilar cleft. A row of basilar cells will remain along the dermis and will have a rounded "tombstone" appearance.4 The superficial dermis may be infiltrated by variable numbers of lymphocytes, plasma cells, and neutrophils. In some areas in the present case, there was a single remaining layer of basilar keratinocytes; however, these cells did not have the classic tombstone appearance, and this was not a consistent feature. There were some rounded keratinocytes around vesicles; however, these were interpreted as apoptotic rather than acantholytic cells. Thus, pemphigus vulgaris was also considered unlikely.
One possible etiology for the lesions observed in this monkey was irritant contact dermatitis. It was speculated that perhaps the isotope may have been passed in urine, and the affected areas (feet and tail) would be the most likely to come into contact with the irritant. The initial gross lesions in irritant contact dermatitis are erythema and papules followed by exudation, scaling and crusting.4 With chronic exposure lichenification, hyperpigmentation, and alopecia can develop.4 The affected sites usually have sparse hair or are hairless due to the protective aspect of the hair-coat. Pruritus can occur but its presence is variable.4 Histologically, the condition is characterized by epidermal necrosis with subepidermal separation and neutrophilic dermatitis. Early in the course of the disease, there may just be epidermal spongiosis and small vesicles. In chronic lesions, there may be parakeratosis, acanthosis, and serous and neutrophilic crusts. There may be variable superficial perivascular mixed inflammation.4 Based on the intraepidermal vesicles, neutrophilic inflammation, irritant contact dermatitis was considered; however, again this does not entirely explain the clinical history and presentation. Ultimately, the disease process in this case is considered a cutaneous drug reaction to the radiolabeled imaging medium. While the histologic features do not entirely fit with the discussed differentials, the presence of vesicular and bullous lesions in combination with the clinical history support this conclusion.
Contributing Institution:
Charles River Laboratories, Mattawan, MI
JPC Diagnosis:
Oral mucosa and haired skin: Epithelial necrosis, coagulative, focally extensive, with subepithelial clefting, ulceration, nuclear streaming of the stratum basale, and subepithelial collagen homogenization and edema.
JPC Comment:
The contributor provides a truly outstanding review of the clinical and histologic features of various forms of cutaneous drug reactions in addition their pathogeneses.
The moderator discussed the importance and value of detailed written descriptions, particularly in regard to the research setting, with this case being a prime example. Given the histologic lesions do not completely meet the criteria of the differentials, the histologic description itself is a useful tool for investigators. In addition, thorough descriptions of what are believed to be already known conditions at the time of writing may in turn retrospectively facilitate the discovery of new entities.
Although unlikely in this case, especially given these cynomolgus macaques were housed indoors, an additional consideration in regard to the development of dermatitis in animals receiving a xenobiotic is cutaneous phototoxicity (i.e. photosensitivity). Cutaneous photosensitivity is a nonimmunologic form of dermatitis induced by cutaneous photoactive agents in the presence of ultraviolet radiation (predominately type A (UVA) light).9 Photosensitivity is a dose-dependent phenomenon with respect to both the photoactive compound and light exposure.5
When exposed to ultraviolet radiation, phototoxic compounds form stable photoproducts that directly cause cell damage or enter an excited state, forming both free radicals and reactive oxygen species that cause direct cell damage and oxidize cellular lipids (e.g. membranes), respectively.5
There are three types of photosensitivity (types I-III), which are classified in regard to the source of the photoactive agent.7
Type I photosensitivity (also known primary photosensitization) predominately occurs as the result of photoactive compounds being ingested, absorbed, and deposited in the skin, although phytophotocontact dermatitis as the result of direct adsorption of compounds into the skin has also been reported. Herbivores are commonly affected as plants are the most common source of ingested photoactive compounds. St. John's wort (Hypericum performatum), Buckwheet (Fagopyrum spp.), spring parsley (Cymopterus watsonii) and bishops weed (Ammi majus) are a few examples of plants containing photoactive pigments.7
Type I photosensitivity may also occur following the administration of medications with photoactive properties. In general, these medications typically have a low molecular weight (200-500 Daltons) and have planar, tricyclic, or polycyclic configurations. However, no elements or configurations automatically impart phototoxicity to compounds. Phototoxic medications include numerous commonly used prescription and over the counter products; one recent report identified 393 medications with evidence of photosensitization, although many medications were historical and are no longer in use today. Medications with high evidence of photosensitization (i.e. ≥15 publications) include (but are not limited to) hydrochlorothiazide, furosemide, amiodarone, naproxen, ketoprofen, piroxicam, lemefloxacin, ciprofloxacin, tetracycline, doxycylcine, griseofulvin, quinine, and promethazine.5
An additional concern in regard to photosensitizing medications is the potential for photocarcinogenic effects, particularly in regard to the development of skin cancer (e.g. squamous cell carcinoma (SCC), basal cell carcinoma (BCC), and melanoma) in humans. Photocarcinogenesis is the subject of controversial scientific discussions and debate as probable mechanisms remain under investigation. The relationship between photosensitive medications and photocarcinogenesis is likely based on multiple factors, including patient age, susceptibility to solar radiation, cumulative dose of medications, and other unknown factors. Psoralens (furocomarins) are associated with the strongest evidence for photocarcinogenic effects; these substances have been investigated in animal and human models, with studies demonstrating increased risk of SCC, BCC, and melanoma. However, multiple medications have been reported to be associated with an increased risk of skin cancer in humans, including NSAIDs and fluroquinolones, thiazide diuretics, tetracyclines, amiodarone, and voriconazole amongst others.5
Type II photosensitivity occurs as the result congenital enzyme deficiencies that result in the accumulation of photodynamic endogenous pigment in the skin due to abnormal heme-synthesis. Deficiency of uroporphyrinogen III cosynthase is a classic example in bovines that results in the deposition and accumulation of porphyrin pigments in many tissues, including the bone and teeth, as well as the skin. Uroporphyrins in the skin are particularly reactive to UVA radiation. Following activation, these pigments cause the formation of membrane damaging reactive oxygen species either directly and/or potentially via activation of the xanthine oxidase pathway.7
Type III photosensitivity is the most common form of photosensitivity in domestic animals and occurs as the result of impaired hepatic clearance of phylloerythrin as the result of either hepatocellular damage or biliary obstruction. Phylloerythrin is a photoactive substance derived from chlorophyll that is normally formed within the digestive tract, absorbed, transported to the liver via portal circulation, and then excreted in the bile. Hepatotoxic plants such as lanata (Lantana camara) or mycotoxins such as sporidesmin, inhibit the hepatic clearance of phylloerythrin, resulting in its accumulation in the skin and subsequent development of cutaneous lesions.7
Histologic lesions associated with cutaneous photosensitization include coagulative necrosis of the epidermis with variable extension to adnexal structures and the superficial dermis, subepidermal clefts, dermal edema, and swollen or necrotic blood vessels that may demonstrate fibrinoid degeneration with variably present thrombosis. There is typically scant inflammation early in the process; however, the tissue is soon infiltrated by neutrophils.7
References:
1. Allen KP, Funk AJ, Mandrell TD. Toxic epidermal necrolysis in two rhesus macaques (Macaca mulatta) after administration of Rituximab. Comp Med. 2005;55(4):377-381.
2. Bernstein JA, DIdiert PJ. Nonhuman primate dermatology: a literature review. Vet Dermatol. 2009;20(3):145-156.
3. Garman RH, Reed C, Blick DW. Toxic epidermal necrolysis in a monkey (Macaca fascicularis). Vet Pathol. 1979;16:81-88.
4. Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis. 2nd ed. Blackwell Science Ltd; 2005.
5. Hofmann GA, Weber B. Drug-induced photosensitivity: culprit drugs, potential mechanisms and clinical consequences. J Dtsch Dermatol Ges. 2021;19(1):19-29.
6. Kim JM, Kim HJ, Min BH, et al. Bullous pemphigoid-like skin blistering disease in a rhesus macaque. J Med Primatol. 2016;45:206-209.
7. Mauldin EA, Peters-Kennedy J. Integumentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. Vol 1. 6th ed. Louis, MO: Elsevier; 2016:577-580.
8. Mecklenburg L, Romeike A. Recommended diagnostic approach to documenting and reporting skin findings of nonhuman primates from regulatory toxicity studies. Tox Pathol. 2016;44(4):591-600.
9. Ngo MA, Maibach HI. Dermatotoxicology: historical perspective and advances. Toxicol Appl Pharmacol. 2010;243(2):225-238.
10. Palanisamy GS, Marcek JM, Cappon GD, et al. Drug-induced skin lesions in cynomolgus macaques treated with metabotropic glutamate receptor 5 (mGluR5) engative allosteric modulators. Tox Pathol. 2015;43:995-1003.
11. Yage JA. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis: a comparative review. Vet Dermatol. 2014;25:406-e64.