Signalment:
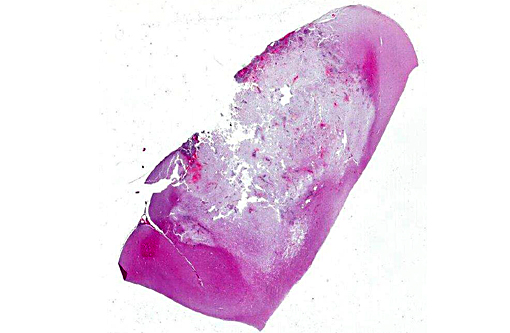
Gross Description:
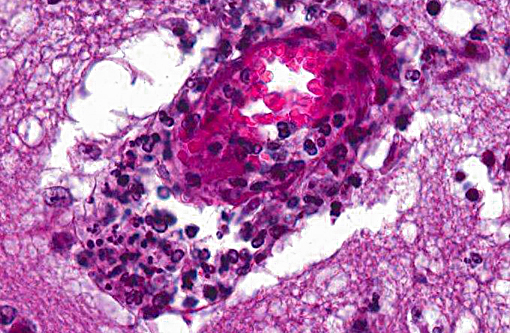
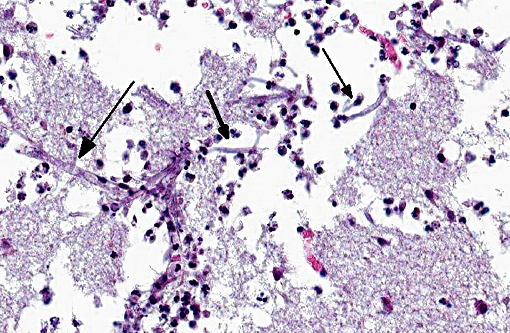
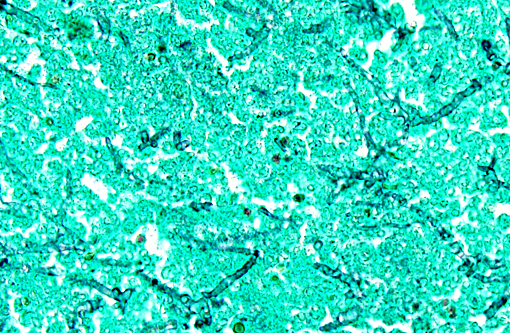
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
However, the exact portal of entry for the fungal infection could not clearly be identified in the present case. It is possible that Aspergillus conidia entered through the perforating wound and germinated within the thoracic cavity, and from there they invaded the blood stream and spread to the lung and central nervous system. But it is also possible the infection route was via inhalation of Apergillus spores, resulting in penetration of distal alveolar spaces. Here, there are optimal environmental conditions for germination into angioinvasive filamentous hyphae that can produce local tissue damage, hemorrhage, infarction, and necrosis.(7)
In healthy, immunocompetent individuals, various elements of the pulmonary innate immune system are involved in recognition and elimination of inhaled Aspergillus conidia, thereby preventing colonization of the respiratory system. Ciliated and mucus secreting epithelial cells perform effective mucociliary clearance that is important for entrapment and elimination of inhaled conidia. Surfactant, mainly produced by type II pneumocytes and Clara cells, has been implicated in antimicrobial activity with surfactant protein A and D serving as collectins. Alveolar macrophages represent first line phagocytic defense by intracellular killing of swollen spores and prevention of germination. Recruited neutrophils play an essential role by extracellular (degranulation) as well as intracellular (phagocytosis) elimination of aspergilli. Dectin-1, expressed by macrophages, neutrophils and dendritic cells, is an important receptor of innate antifungal defense being essential for spore recognition and phagocytosis, as well as production of oxygenated free radicals (fungicide al activity). Above that, certain Toll-like receptors (TLR) have been found to play a predominant role in the recognition of A. fumigatus (TLR2: recognition of spores, TLR4: recognition of spores and hyphae).(8)
On the other hand, several pathogenicity factors were found in different Aspergillus spp. to overcome certain host defense mechanisms such as endotoxins that inhibit epithelial ciliary activity, as well as a variety of proteases (including elastase, collagenase and trypsin) that damage epithelial cells and, thus, impair effective mucociliary clearance.(1,5) Furthermore, A. fumigatus produces a phospholipid capable of decreasing the binding of complement factor C3b to its surface, resulting in disturbed complement activation.(7) Also other fungal proteins of A. fumigatus are probably related to virulence by promoting mycelial growth in lung parenchyma or structural alterations of conidia that are resistant to host defense mechanisms.(1)
Moreover, it is likely that Aspergillus mycotoxins can work as virulence factors due to direct cytotoxic effects. In vitro studies revealed that aflatoxin (produced by A. fumigatus) suppresses the function of macrophages, and ochratoxin (produced by A. ochraceus) is cytotoxic to lymphocytes and suppresses lymphocytic, monocytic and granulocytic activity. As other possible immunosuppressive mycotoxins, gliotoxin, fumagillin, fumigacin, fumitremorgin A and Asp-hemolysin are discussed while different mycotoxins together may have synergistic effects. However, further in vivo studies are needed for confirmation of direct relation to Aspergillus pathogenesis.(6) Beyond that, melanin pigment, mannitol, catalases and superoxide dismutases are suggested as antioxidant defenses produced by Aspergillus.(4) Although it seems that certain antioxidant molecules produced by A. fumigatus do not directly inhibit the oxidizing activity of phagocytes, inhibition of reactive oxygen species production by macrophages (e.g. with high blood cortisol levels or corticosteroid treatment) abolishes their ability to kill the spores while phagocytosis continues so that conidia can germinate and proliferate intracellularly.(8)
However, since pulmonary macrophages and neutrophils constitute a crucial part of first line innate host defense, neutropenia and long-term corticosteroid treatment or hyperglucocorticoidism, as observed in the present case, are generally regarded as major risk factors for the pathogenesis of invasive aspergillosis.(1,4)
JPC Diagnosis:
Conference Comment:
Aspergillosis is perhaps more readily recognized as the cause of granulomatous pneumonia and air sacculitis in avian species, mycotic rhinitis in dogs, abortion in cattle, secondary abomasal ulcers in ruminants following grain overload or mastitis, and hepatocyte megalocytosis and necrosis in dogs.(2,3) The latter is associated with production of aflatoxin of which there are several produced by Aspergillus spp. with B1 being the most significant and best studied example.(10) Toxin production tends to be greatest in stored or unharvested mature grains. Among nonhuman primates, reports of infection are seemingly rare, limited to a single outbreak at the London Zoo in conjunction with tuberculosis. During this outbreak, Old World monkeys were affected by disseminated lesions in the lungs, liver, kidneys and spleen.(9)
References:
1. Al-Alawi A, Ryan CF, Flint JD, et al. Aspergillus-related lung disease. Can Respir J. 2005;12(7):377-387.
2. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier Saunders; 2007:229.
3. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier Saunders; 2007:640.
4. Dagenais TRT, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22:447-465.
5. Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26(4):781-803.
6. Kamei K, Watanabe A. Aspergillus mycotoxins and their effect on the host. Med Mycol. 2005;Suppl43:S95-S99.
7. Latg+�-� JP. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev.1999;12(2):310-350.
8. Mansour MK, Tam JM, Vyas JM. The cell biology of the innate immune response to Aspergillus fumigatus. Ann NY Acad Sci. 2012;DOI: 10.1111:78-84.
9. Simmons J, Gibson S. Bacterial and mycotic diseases of nonhuman primates. In: Abee CR, Mansfield K, Tardiff S, Morris, T, eds. Nonhuman Primates in Biomedical Research: Diseases. 2nd ed. Vol. 2. San Diego, CA: Elsevier Inc. 2012:156-157.
10. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmers Pathology of Domestic Animals. 5th ed. Vol. 2. Philadelphia, PA: Elsevier Saunders; 2007:370-371.