Signalment:
Gross Description:
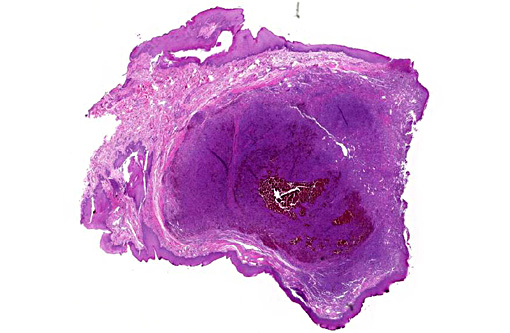
Histopathologic Description:
Morphologic Diagnosis:
Condition:
Contributor Comment:
Epulis is a non-specific term referring to a benign local exophytic growth of the oral mucosa.(2) Epulides are common in the dog (up to 59% of benign canine oral neoplasms), and canine epulides can be divided into reactive lesions (giant cell epulis, fibrous epulis, pyogenic granuloma and reactive exocytosis) and peripheral odontogenic tumors (fibromatous epulis of the periodontal ligament, acanthomatous epulis (acanthomatous ameloblastoma) and calcifying epithelial odontogenic tumor (amyloid producing odontogenic tumor)).(5,11)
Giant cell epulis (GCE) was reported in a dog as early as 1917 but is a rare lesion, with little information available in the veterinary literature.(4-6,11,12) Classified under Tumor-like Lesions in the WHO Histologic Classification of Tumors of the Alimentary System of Domestic Animals, GCE is thought to represent a benign hyperplastic lesion rather than a true neoplastic process.(6,11,12) Despite the low number of documented cases, a variety of canine ages and breeds have been reported, with ages ranging from 1 to 11 years.(5,7,12) These masses arise from gingiva, most often adjacent to the maxillary and mandibular premolars and are typically red to purple and lobulated.(11,12)
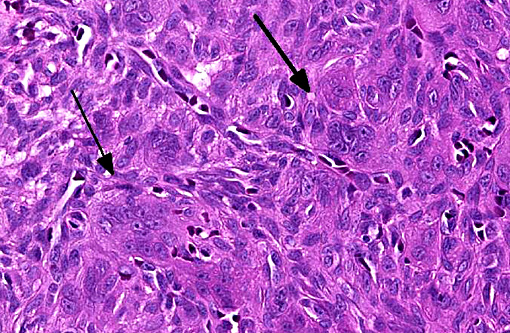
Histologic features of canine and feline GCE are consistent with those reported for human patients.(4) GCE typically presents as a nonencapsulated, poorly demarcated vascular submucosal nodule covered by hyperplastic gingival epithelium that may be ulcerated.(1,2,5,11) Nodules consist of large numbers of randomly distributed multinucleated giant cells in a background of mononuclear stromal cells.(1,7-11) Multinucleated giant cells are irregularly shaped, often containing 10-20 visible nuclei with abundant eosinophilic cytoplasm, and may vary with respect to size (up to and exceeding 100um diameter), amount of cytoplasm, nuclear chromatin pattern and prominence of the nucleolus.(1,2,6-8,10,11) Within the giant cell population, cellular and nuclear pleomorphism are not seen and there are few to no mitoses.(2,4,11) The stroma is typically well vascularized and highly cellular, containing numerous round and spindle shaped mononuclear cells that exhibit rare mitoses.(1,2,5-8,10,11) The stroma shows varying degrees of mixed inflammation, as well as frequent hemorrhage, hemosiderosis and occasional osteoid or woven bone deposition.(1,2,5,7,8,10,12)
In the dog, GCE is reported to exhibit slow growth without invasion or metastasis.(4,6,11) Several references have reported no recurrence following removal (post-excision follow-up time ranged from 8-18 months).(5,11,12) In a retrospective study of 52 feline epulides by deBruijin et al (2007), GCE was the second most common type of epulis diagnosed, after fibromatous epulis.(2) In contrast to the behavior of canine GCE, the feline GCE in this study exhibited rapid growth and an aggressive clinical course, with seven out of thirteen recurring within two months of marginal excision.(2) The authors hypothesized that the high recurrence rate and poor prognosis of the GCE in the cat after marginal excision alone may be related to the rapid growth and poor demarcation of the lesion associated with the persistent inflammatory component.(2)
The origin of the multinucleated giant cells in GCE remains unknown. Ultrastructural and immune studies have shown the giant cells to be derived from macrophages, though they are not functional in terms of phagocytosis and bone resorption.(1,11) Due to the close association of GCE with bone, it has been suggested that giant cells are of osteoclast origin, though others have proposed that multinucleated giant cells form by fusion of infiltrating macrophages, as the stroma may contain chronic granulomatous inflammation.(2,4,12) Immunohistochemical studies performed by deBruijn ND et al (2007) indicated that giant cells were strongly positive for vimentin and an osteoclast marker (tartrate-resistant acid phosphatase, TRAP) and negative for factor VIII.(2) Giant cell cytoplasm was also positive for the polyclonal antibody RANK, a cytokine leading to the differentiation of osteoclast progenitors into mature osteoblasts in the presence of its ligand (RANKL).(2) Therefore, the authors hypothesized that the giant cells are most likely formed from a monocyte/macrophage-like osteoclast precursor that differentiates into osteoclasts under the influence of mononuclear osteoblast-like stromal cells.
In humans, this lesion has been known by a variety of names, including peripheral giant cell tumor, peripheral giant cell granuloma, reparative giant cell granuloma, giant cell hyperplasia of the oral mucosa and osteoclastoma.(1,8,10) It accounts for approximately 1% of all oral pathologic lesions and occurs in all age groups, with the highest incidence in the fourth to sixth decade of life and the second highest incidence in the first to second decades.(1,8,9,11) It is generally accepted that this mass represents a benign hyperplastic lesion rather than a true neoplastic process, and it is known that chronic trauma or irritation can induce produce granulation tissue with chronic inflammation and fibroblast proliferation and manifest as reactive hyperplasia.(1,9-11) However, the inciting cause of GCE is not known and is controversial, with proposed causes including tooth extraction, poor dental restorations, dental malpositioning, ill-fitting dentures, plaque, calculus or food impaction.(1,8-11)
The lesion originates from the connective tissue of the periosteum (mucoperiosteum or periodontal ligament) or from the periodontal membrane and is localized in the interdental papilla, edentulous alveolar margin or at the marginal gingival level.(1,8,10) The mass may involve the mandible or maxilla, though mandibular involvement is approximately 2.5 times more frequent than maxillary, and the premolar and anterior molar regions are most often affected.(1,8-11) Masses vary in size (though rarely exceed 2cm in diameter), are dark red to purple to blue, and can be polypoid, nodular or sessile.(1,8,10) The consistency of the mass depends on age of lesion because as time passes, an increased collagen component leads to increased firmness, and ulceration and bleeding can occur secondary to trauma.(1,8,10)
Behavior is variable, with growth ranging from silent to aggressive, affecting adjacent tissues by direct spread, though underlying bone is rarely affected.(1,9) The mass is not usually painful unless there is mucosal ulceration or erosion of the underlying bone, though swelling and dental mobility is common.(1,9,10) Occasional cases in children have been documented to be larger and more aggressive, with local bone destruction, displacement of the adjacent teeth and multiple recurrences.(1,9) Wide surgical excision with extensive clearing of the base of the lesion is usually curative, though when the periodontal membrane is affected, extraction of adjacent teeth may be necessary for full removal.(1,10,11) If resection is only superficial, recurrence is possible, though infrequent (5-11%).(1,8,10,11)
An interesting differential diagnosis in humans is brown tumor; a rare, non-neoplastic reactive growth associated with primary, secondary and/or tertiary hyperparathyroidism.(1,7-10) These masses are solitary or multiple, most often seen on the maxilla, ribs, clavicles and pelvic bones, and the name brown tumor is attributed to the gross brown discoloration of the affected tissue due to excessive hemorrhage and hemosiderosis.(6) The growth appears centrally in bone but can perforate the cortical layer, spreading towards the soft tissues, and cannot be distinguished from giant cell epulis based on histology.(1,10) However, this condition is often suspected when there are multiple lesions and recurrences despite adequate treatment.(1,9,10) Interestingly, a report by Headley et al (2008) described the occurrence of an oral lesion with features consistent with brown tumor in a 14-month-old English bulldog with renal secondary hyperparathyroidism due to chronic renal insufficiency.(7)
JPC Diagnosis:
Conference Comment:
A recent publication followed 26 diagnosed peripheral giant cell granulomas and concluded they are benign and rarely recur with even marginal surgical excision. Additionally, the number of giant cells and mitotic index of the lesions do not correlate with biologic behavior.(3)
References:
1. Chaparro-Avendano AV, Berini-Aytes L and Gay EC: Peripheral giant cell granuloma. A report of five cases and review of the literature. Med Oral Patol Oral Cir Bucal 10: 48-57, 2005.
2. DeBruijn ND et al: A Clinicopathological Study for 52 Feline Epulides. Vet Pathol 44: 161-169, 2007.
3. Desoutter AV, Goldschmidt MH, Sanchez MD. Clinical and histological features of 26 canine peripheral giant cell granulomas (formerly giant cell epulis). Vet Pathol. 2012;49(6):1018-1023.
4. Forman J and Reed CI: A Fibro-blastoma of the alveolar Border of the Jaw Containing Giant cells (a Giant Cell Epulis). Ohio J Sci 17(1): 1-5, 1917.
5. Gardner DG: Epulides in the dog: a review. J Oral Pathol Med 25: 32-37, 1996.
6. Head KW et al. Histological Classification of Tumors of the Alimentary System of Domestic Animals. 2nd Series, World Health Organization International Histological Classification of Tumors of Domestic Animals, 2003.
7. Headley SA et al: Oral lesions associated with renal secondary hyperparathyroidism in an English bulldog. Semina: Ciencias Agrarias, Londrina. 29(2): 407-412, 2008.
8. Rey JMG et al: Peripheral giant-cell granuloma. Review of 13 cases. Medicina Oral 7: 254-259, 2002.
9. Ruiz BD et al: Reparative giant cell granuloma in a pediatric patient. Med Oral Patol Oral Cir Bucal 12: E331-335, 2007.
10. Shadman N, Ebrahimi SF, Jafari S and Eslami M: Peripheral Giant Cell Granuloma: A Review of 123 Cases. Dent Res J 6(1): 47-50, 2009.
11. Valentine BA and Eckhaus MA: Peripheral Giant Cell Granuloma (Giant Cell Epulis) in Two Dogs. Vet Pathol 23: 340-341, 1986.
12. Yoshida K et al: Clinicopathological Study of Canine Oral Epulides. J Vet Med Sci 61(8): 897-902, 1999.