CASE III: EX57_19 (JPC 4152810)
Signalment:
2-years-old, male, inland bearded dragon (Pogona vitticeps)
History:
The bearded dragon was referred with a history of anorexia and jaundice. The reptile died spontaneously before therapy and a complete necropsy was performed by the referring veterinarian. The entire liver was submitted for histopathology.
Gross Pathology:
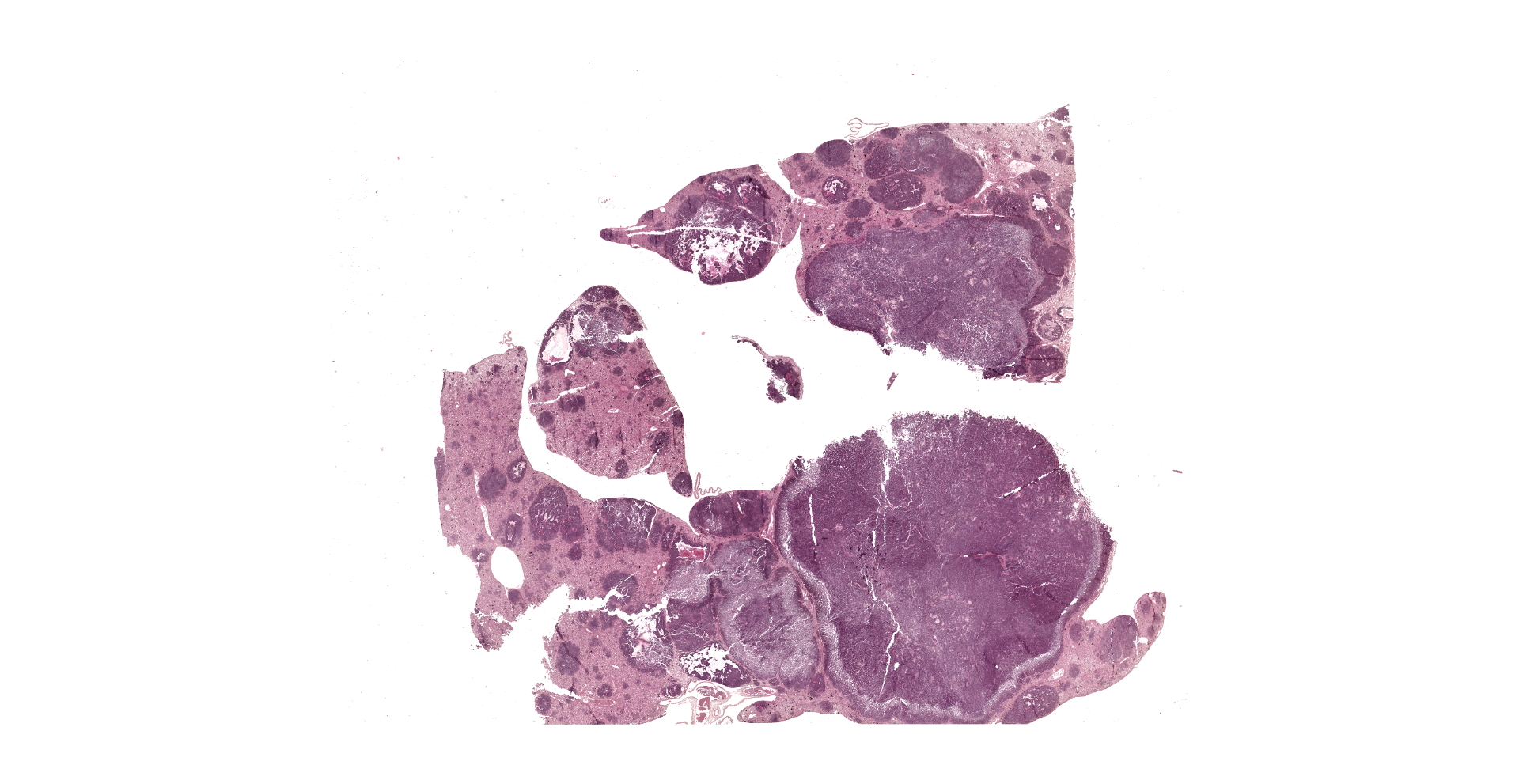
The liver was severely enlarged (9x5.5x2 cm) and characterized by diffuse yellowish discoloration and by multifocal to coalescing, white-yellowish nodules bulging from the surface and ranging from 0.5 up to 2 cm in diameter.
Laboratory Results:
None provided.
Microscopic Description:
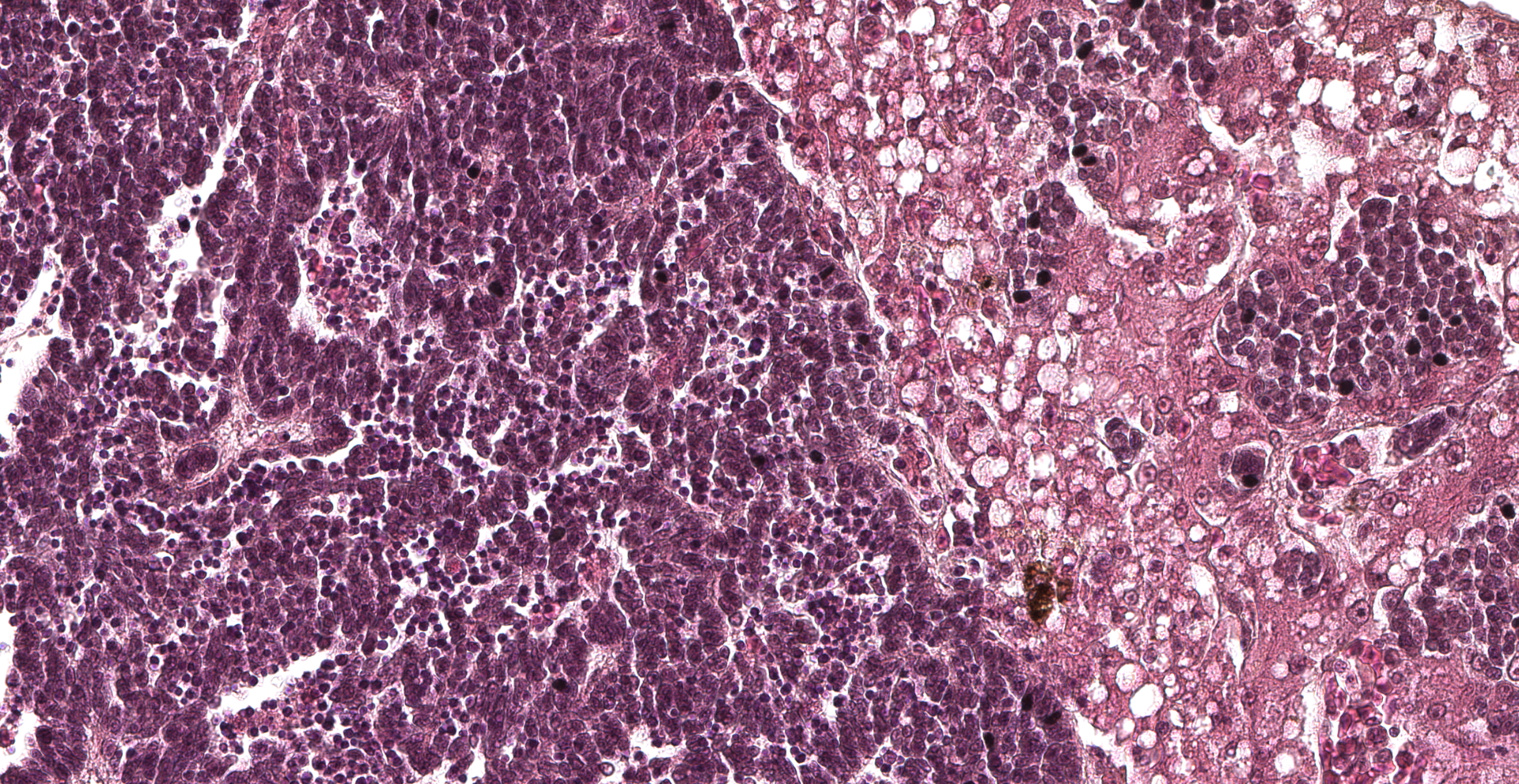
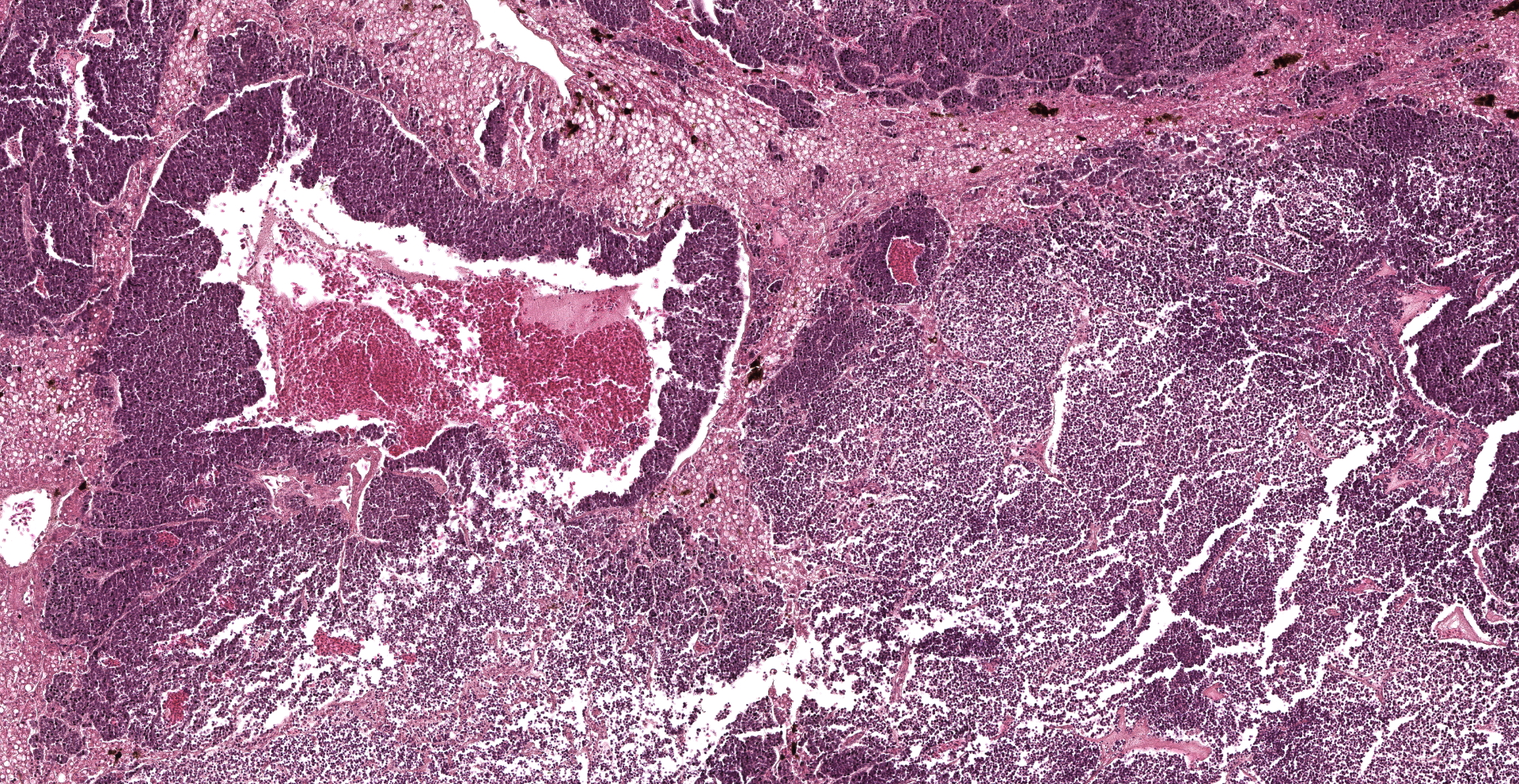
Approximately 70% of the hepatic parenchyma is substituted by multiple nodules ranging from 350 µm to 10 mm in diameter and composed of a multifocal to coalescing, variably demarcated, unencapsulated, densely cellular neoplasms with expansile to infiltrative growth patterns. Neoplastic nodules consist of cells arranged in palisades, nests, rosettes, and pseudorosettes, supported by a fine fibrovascular stroma. Neoplastic cells are polygonal, 15-20 microns in diameter, with variably distinct margins and high nuclear-cytoplasmic ratio. Cytoplasm is scant, finely granular, and weakly eosinophilic. The nucleus is paracentral, oval to round, 10-15 microns, with granular to marginated chromatin, and 1-2 basophilic, paracentral, 2-3 microns prominent nucleoli. Anisocytosis and anisokaryosis are moderate. Mitotic figures range from 0 to 3/HPF and are often atypical. Numerous apoptotic cells and occasional bi- and tri-nucleated cells are also present. The centre of some of the nodules is diffusely necrotic with variable amount of fibrin. Some neoplastic cells impinge and infiltrate vascular walls producing endoluminal projections. Scattered in the lumen of liver sinusoids, neoplastic emboli are also detectable. Viable hepatocytes bordering neoplastic nodules are atrophic, in association with rows of hepatocytes characterized by lipidosis. Mild to moderate, diffuse hyperplasia and hypertrophy of the melanomacrophagic centres is evident in normal hepatic parenchyma.
Contributor's Morphologic Diagnoses:
Liver. Neuroendocrine carcinoma.
Contributor's Comment:
Neuroendocrine carcinomas ("carcinoids") are rare neoplasms arising from neuroendocrine cells residing in several organs but most commonly develop in the gastrointestinal and respiratory tracts.6 Neuroendocrine carcinomas have been reported in dogs and cats,5 as well as occasionally in cattle4,10 and horses.9,13
Captive reptiles have an incidence of neoplasia comparable to that of mammals and birds.3 Snakes are the most commonly reported class, followed by lizards, chelonians, and crocodilians.3 In lizards, hematopoietic, cutaneous, and hepatic systems are the most frequently affected by neoplasia, with bearded dragons (Pogona vitticeps) and green iguanas (Iguana iguana) being over-represented in recent years, likely based on their increasing popularity as pets.3 Lymphoma, squamous cell carcinoma, and peripheral nerve sheath tumors are among the most common neoplasms described in bearded dragons.3,16 Few cases of gastric neuroendocrine carcinomas have been described in young animals of this species.1,7,8,14,16
In this case the liver was the only tissue submitted. Neuroendocrine carcinomas primary developing in the liver are rare.17 The angiocentric organization of neoplastic nodules observed in the current case suggests a metastatic nature of the neoplasm. Indeed, liver is a common site of metastasis of gastric neuroendocrine carcinomas in bearded dragons.1,7,8,14,16 Other common sites for metastasis are the kidneys, followed by other organs like pancreas, intestine, heart, oviduct, ovary, lungs, adrenal gland, and spleen.1,16
Clinical sings commonly associated with neuroendocrine carcinomas in bearded dragons include anorexia, weight loss, weakness, and vomiting, occasionally combining with clinicopathological abnormalities like anemia and hyperglycemia.1,7,14,16
Neuroendocrine carcinomas are easily recognized by the typical histological pattern of palisading nests, solid masses, or anastomosing ribbons of cells separated by a thin fibrovascular stroma, with occasional formation of rosettes or acinar-like structures containing eosinophilic material.11 In humans, neuroendocrine neoplasms have been historically classified using a range of site-specific terminologies and criteria, with consequent confusion among pathologists.15 In order to reduce inconsistencies and contradictions in the classification scheme, the WHO recently introduced the distinction between differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs), supported by genetic evidence at specific anatomic sites as well as clinical, epidemiologic, histologic, and prognostic differences.15 However, too few cases have been observed to date in domestic animals to determine the most appropriate prognostic and other features for these neoplasms.11
Further subclassification of neuroendocrine tumors can be based on hormone production by neoplastic cells and the presence or absence of a clinical syndrome related to hormone secretion.16 Indeed, these neoplasms may be non-functional, secrete a single product, or exhibit multihormonal expression.16 In domestic animals, functional neuroendocrine carcinomas have been reported in the intestinal tract of dogs.11 Immunohistochemistry provides the gold standard for confirmation of neuroendocrine origin and for functional subclassification based on detectable secretory products.16 Markers to determine neuroendocrine differentiation include antibodies against secretory granule proteins (i.e. chromogranins A and B), synaptic vesicle proteins (i.e. synaptophysin), and enzymes (i.e. neuron-specific enolase) specific for neuroendocrine cells.16 However, staining for these generic markers has been rarely reported in bearded dragon carcinoids with the exclusion of somatostatin that is the most frequent marker utilized and observed in these tumors.1,7,8,16 This has led to the final diagnosis of somatostatinoma for all carcinoids reported in bearded dragons, this observation being consistent with the hyperglycemia occasionally observed and resembling what reported in human somatosostatinomas.14
Furthermore, the primary gastrointestinal location of the somatostatinomas in bearded dragons is reminiscent of those associated with neurofibromatosis type 1 (NF-1, also called Von Recklinghausen's disease) in humans.7,16 Indeed, neuroendocrine carcinomas associated with NF-1 show decreased expression of neurofibromin. This feature was also observed by Ritter et al. (2009)16 in all the bearded dragon carcinoids examined by immunohistochemistry, possibly suggesting that a decrease in functional neurofibromin protein may be involved in the pathogenesis of neuroendocrine carcinomas, and specifically somatostatinomas, in this species.
Contributing Institution:
JPC Diagnosis:
1. Liver: Neuroendocrine carcinoma.
2. Liver, hepatocytes: Lipidosis, diffuse, marked.
JPC Comment:
The contributor provides an excellent review neuroendocrine tumors in domestic and exotic species, a broad subset of neoplasms that arise from cells within the diffuse endocrine system found throughout the body, predominantly in the gastrointestinal and respiratory tracts.12
Although the neuroendocrine tumor in this case likely occurred as the result metastatic disease, primary neuroendocrine tumors may also arise in the liver and biliary tree. Previously referred to as "carcinoids", these neoplasms are believed to originate from the diffuse neuroendocrine cell population within the biliary epithelium or from hepatic progenitor cells, also known as oval cells.12
One retrospective study12 of 13 cases of primary canine gallbladder neuroendocrine carcinoma found Boston terriers to be overrepresented, suggesting a possible breed predilection. Common findings amongst the cohort were emesis, often in conjunction with hematemesis, and elevated liver enzymes with 100% demonstrating elevated alanine aminotransferase and alkaline phosphatase activity. Histologically, all 13 cases shared similar features as previous described by the contributor, in addition to positive immunoreactivity for neuron specific enolase, chromogranin A, synaptophysin, and gastrin. Greater than 50% of neoplastic cells in every case demonstrated immunoreactivity for synaptophysin, with similar results for gastrin (12/13 cases); 25-50% of neoplastic cells were immunopositive in the one outlying case. Chromogranin immunoreactivity was reported in each case, ranging from <25% immunoreactivity to >50%. As with primary neuroendocrine tumors arising in other locations, vascular invasion and metastasis were common, identified in 61.5% and 43% of the cases, respectively.12
The correlation between emesis and hematemesis in addition to 100% of canine gallbladder neuroendocrine carcinomas demonstrating strong immunopositivity for gastrin was strongly suggestive of a paraneoplastic syndrome, variants of which are further discussed below. Interestingly, gastrin secretion by gallbladder neuroendocrine carcinomas is associated with a better prognosis in humans (MST 3.7 years) compared to hepatic neuroendocrine carcinomas (MST 3 days). This disparity is likely multifactorial, however, early tumor identification as a result patients seeking treatment for paraneoplastic syndrome associated symptoms is likely a significant contributing factor.12
A historical review of neuroendocrine tumors begins in 1870 with German physiologist Rudolf Heidenhain being the first note a group of gastrointestinal cells with "yellow" chromate staining properties that were distinctly different from chief, parietal, and enteric cells. Nikolai K. Kultschitzky, a Russian anatomist and histologist who later become the education minister of the Russian empire, further increased awareness of these cells 1897, for which they became known as "Kultschitzky's cells". The term "enterochromaffin" was introduced by Carmelo Ciaccio in 1907 and Harry Kull noted these cells demonstrated a similar morphology to chromaffin cells in 1925. In 1914, Antonin Gosset and Pierre Masson, a French surgeon and French-Canadian pathologist, respectively, used silver impregnation techniques to demonstrate the argentaffinity (i.e. argyrophilia) of carcinoid tumors and suggested they may arise from enterochromaffin cells.2
The term "carcinoid" was introduced by Siegfried Oberndorfer, a German pathologist, in 1907 in a report describing four pea-sized, seemingly benign "Karzinoide Tumoren" in the ileum of a woman that died from tuberculosis. Notably, German pathologists Theodor Langhans and Otto Lubarsch likely described similar lesions but did not recognize them as distinct entities decades earlier in 1867 and 1888, respectively. Two decades after "discovering" carcinoids, Oberndorfer reversed his earlier opinion of these lesions being benign, reporting evidence of metastasis as an indication of malignancy. However, the significance of this finding faded over time, largely due to Oberndorfer's departure from Germany as a result of his ethnic background prior to World War II. Although his research continued at the University of Istanbul in Turkey, his publications were printed in Turkish and failed to gain significant attention in the scientific community. As a result, misconceptions of the malignant behavior of carcinoids remained for several decades. In 1995, Italian pathologist Carlo Capella and other neuroendocrine tumor experts revised the classification scheme of neuroendocrine tumors and suggested the term "carcinoid tumor" no longer be applied and instead use the term "neuroendocrine tumor".2
Functional neuroendocrine tumors are commonly associated with paraneoplastic syndromes and include entities such as gastrinoma, insulinoma, glucagonoma, cholecystokininoma, and somatostatinoma.2
In 1955, US Surgeons Robert Zollinger and Edwin Elson described two cases of severe peptic ulcers that were associated with pancreatic neuroendocrine tumors. Three years later, gastrin was extracted from a pancreatic neuroendocrine tumor. Zollinger-Ellison syndrome has since been linked to gastrinomas.2
Cholecystokinin (CCK) is normally produced by specialized I cells in the gastrointestinal tract. Cholecystokininoma syndrome is characterized by nonwatery diarrhea, severe weight loss gall bladder, and peptic ulcers. Interestingly, peptic ulcers associated with cholecystokinomas occur due to CCK being a full agonist for the gastrin/CCK-B receptor. Therefore plasma gastrin concentrations can be used to differentiate between gastrin and CCK secreting pancreatic tumors associated with peptic ulcers, which should not be elevated in cases of cholecystokininomas.2
US surgeon Allen Whipple and pathologist Kneeland Frantz identified key features of insulinomas (also known as 'Whipple's triad') to be 1) symptoms known or likely to be caused by hypoglycemia, 2) hypoglycemia measured at the time of symptoms, and 3) relief of symptoms upon return to normoglycemia.2
Glucagonomas typically arise in the pancreas and are associated with hyperglycemia.2
Somatostatinomas most commonly arise from the pancreas or the proximal duodenum in the periampullary region and are associated with diabetes mellitus, cholithiasis, weight loss, steatorrhea, and diarrhea.2
References:
1. Anderson KB, Meinkoth J, Hallman M, Bailey K, Brandão J. Cytological diagnosis of gastric neuroendocrine carcinoma in a pet inland bearded dragon (pogona vitticeps). J Exot pet Med. 2019;29:188?193.
2. de Herder WW, Rehfeld JF, Kidd M, Modlin IM. A short history of neuroendocrine tumours and their peptide hormones. Best Pract Res Clin Endocrinol Metab. 2016;30(1):3-17.
3. Christman J, Devau M, Wilson-Robles H, et al. Oncology of reptiles: diseases, diagnosis, and treatment. Vet Clin Exot Anim Pract. 2017;20:87?110.
4. Johnson LK, Nunez A, Bracegirdle JR, Dwyer JR, Konold T. Neuroendocrine carcinoma of the liver and gallbladder in a cow. J Comp Pathol. 2008;138:165?168.
5. Kita C, Yamagami T, Kinouchi S, et al. A feline case of hepatic neuroendocrine carcinoma with gastrin immunoreactivity. J Vet Med Sci. 2014;76:887?890.
6. Landen S, Elens M, Vrancken C, Nuytens F, Meert T, Delugeau V. Giant hepatic carcinoid: a rare tumor with a favorable prognosis. Case Rep Surg. 2014;2014.
7. Levine BS, Mammal EC. Gastric endoneurocrine carcinoma (somatostatinoma) in a bearded dragon, Pogona vitticeps. Proc Assoc Reptil Amphib Vet. 2011;18:149?152.
8. Lyons JA, Newman SJ, Greenacre CB, Dunlap J. A gastric neuroendocrine carcinoma expressing somatostatin in a bearded dragon (Pogona vitticeps). J Vet diagnostic Investig. 2010;22:316?320.
9. Van Maanen C, Klein WR, Dik KJ, Van den Ingh T. Three cases of carcinoid in the equine nasal cavity and maxillary sinuses: histologic and immunohistochemical features. Vet Pathol. 1996;33:92?95.
10. Michishita M, Takahashi K, Moriya H, Nakamura S, Koyama H, Sako T. Poorly differentiated rectal carcinoid in a cow. Vet Pathol. 2007;44:414?417.
11. Munday JS, Löhr C V, Kiupel M. Tumors of the alimentary tract. Tumors Domest Anim. 2016;499?601.
12. O'Brien KM, Bankoff BJ, Rosenstein PK, Clendaniel DC, Sánchez MD, Durham AC. Clinical, histopathologic, and immunohistochemical features of 13 cases of canine gallbladder neuroendocrine carcinoma. J Vet Diagn Invest. 2021;33(2):294-299.
13. Orsini JA, Orsini PG, Sepesy L, Acland H, Gillette D. Intestinal carcinoid in a mare: an etiologic consideration for chronic colic in horses. J Am Vet Med Assoc. 1988;193:87?88.
14. Perpiñán D, Addante K, Driskell E. Gastrointestinal Disturbances in a Bearded Dragon (Pogona vitticeps). J Herpetol Med Surg. 2010;20:54?57.
15. Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31:1770?1786.
16. Ritter JM, Garner MM, Chilton JA, Jacobson ER, Kiupel M. Gastric neuroendocrine carcinomas in bearded dragons (Pogona vitticeps). Vet Pathol. 2009;46:1109?1116.
17. Zhao ZM, Wang J, Ugwuowo UC, Wang L, Townsend JP. Primary hepatic neuroendocrine carcinoma: report of two cases and literature review. BMC Clin Pathol. 2018;18:3.