Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 1
August 23rd, 2017
CASE III: RP22064 (JPC 4066355).
Signalment: Adult, female, brush rabbit (Sylvilagus bachmani).
History: This animal was 1 of 2 rabbits found dead in a lion exhibit in the same week.
Gross Pathology: The rabbit had small adipose stores. There were fleas in the haircoat. The lungs were red but floated in formalin.
Gross Morphologic Diagnosis: None provided.
Laboratory results: Toxoplasma gondii was confirmed by PCR and sequencing performed on tissues from the second brush rabbit found in the same enclosure.
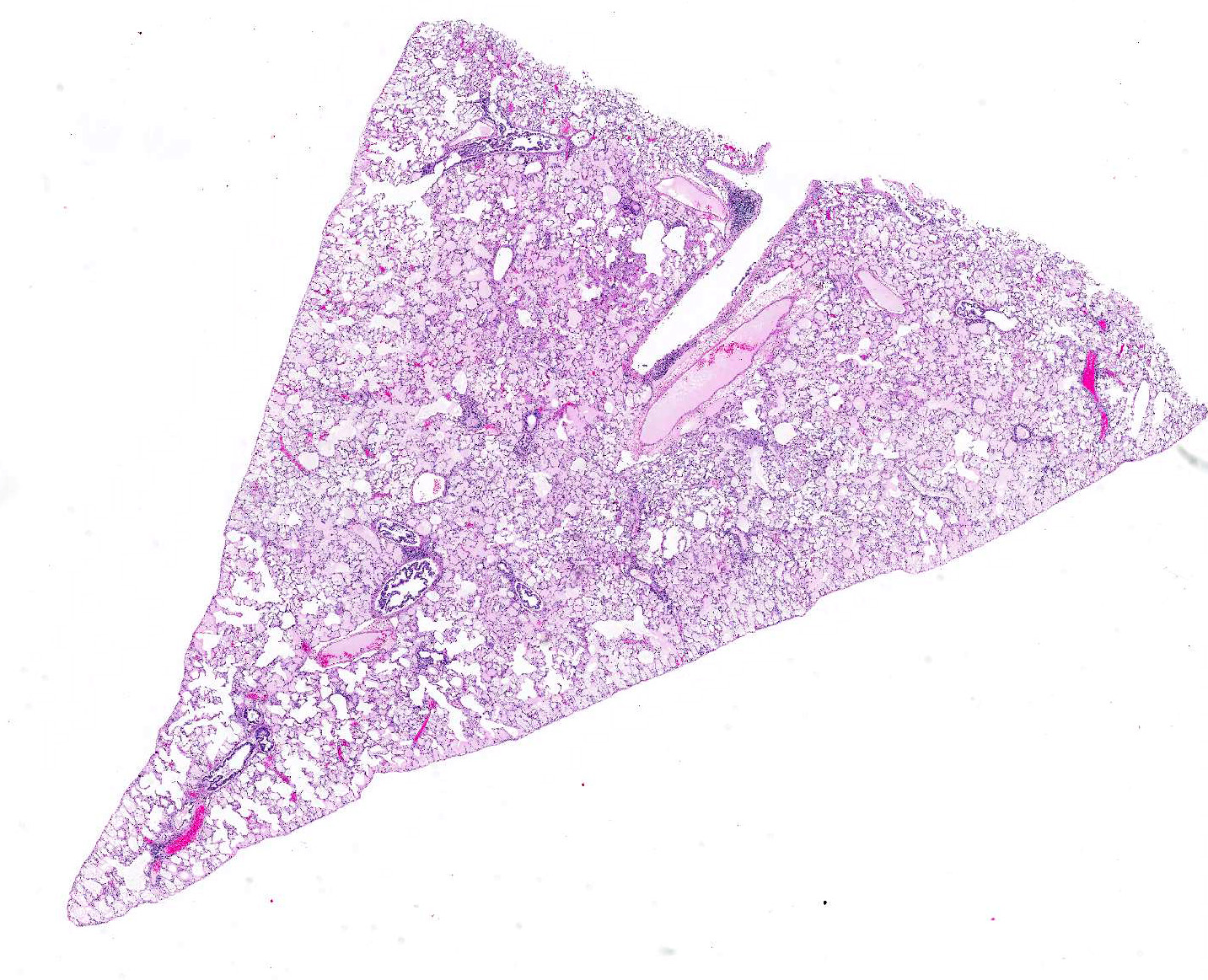
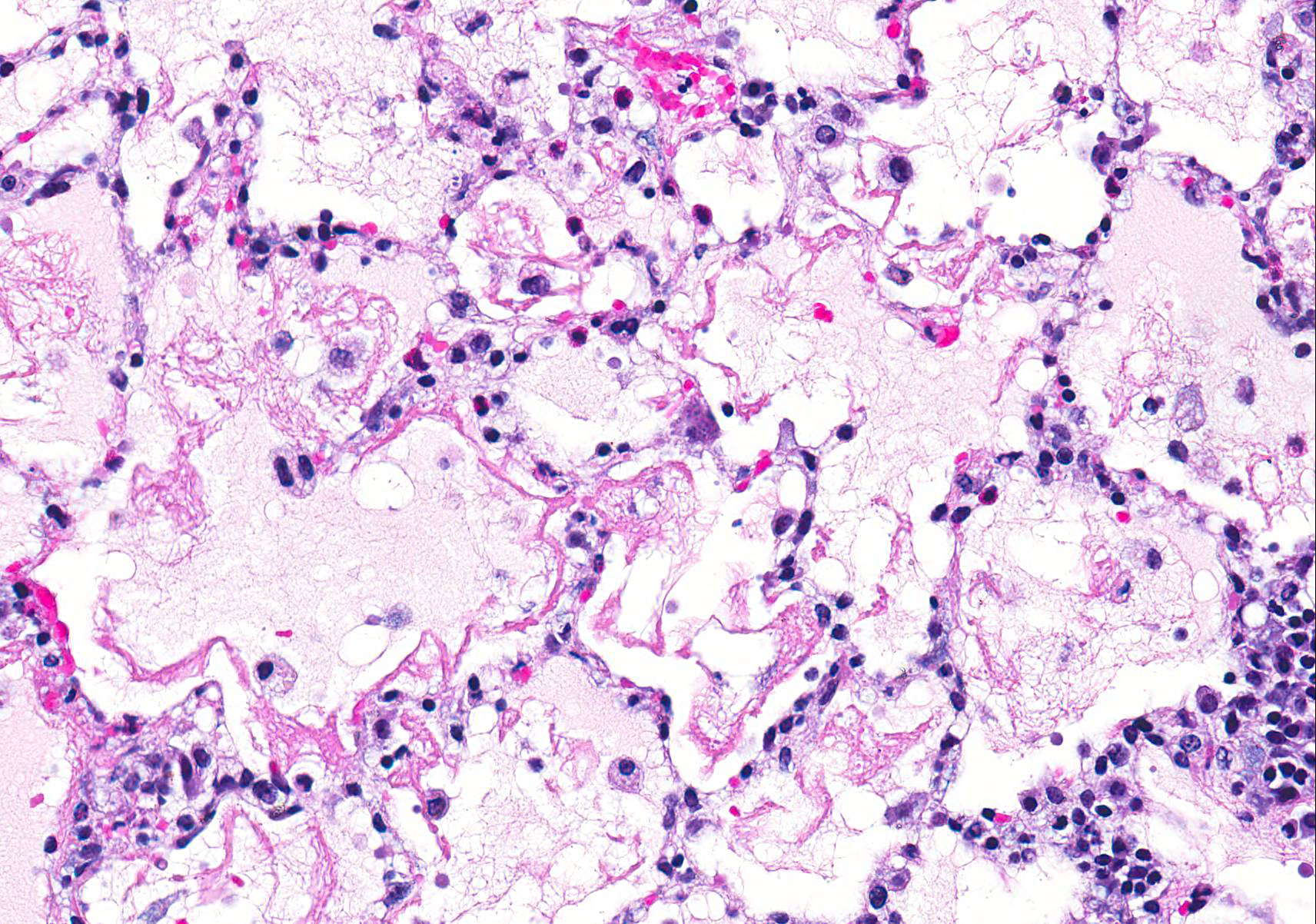
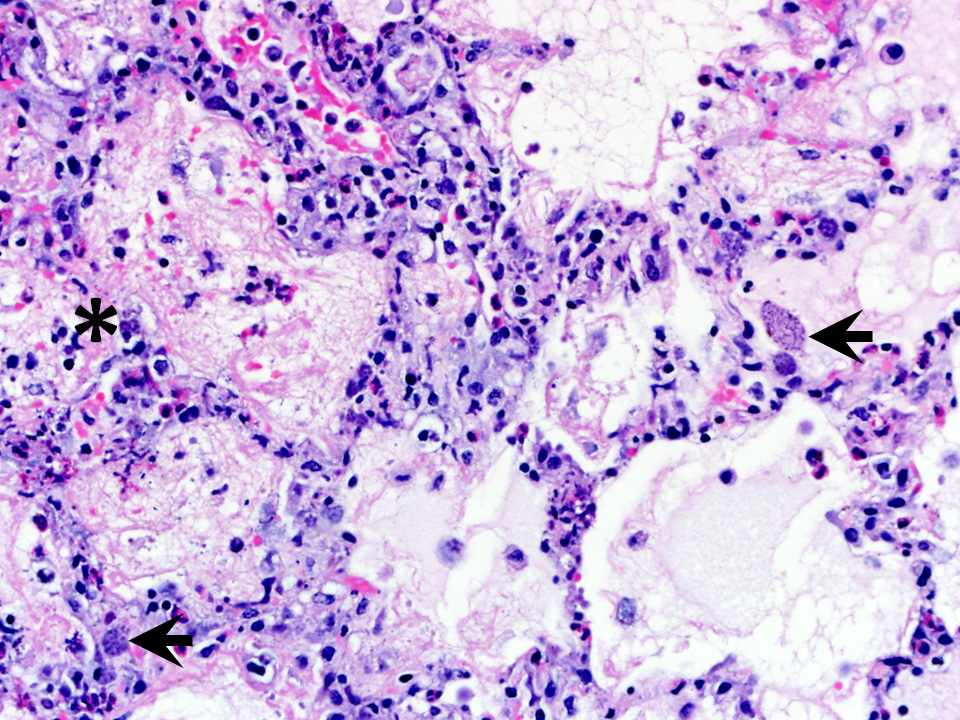
Microscopic Description: Examined is a section of lung in which alveolar lumina are diffusely flooded with proteinaceous fluid (edema) and beaded eosinophilic fibrillar material (fibrin) admixed with plump and foamy alveolar macrophages, fewer lymphocytes, nondegenerate heterophils and plasma cells and scant hemorrhage. Small amounts of fibrin and few lymphocytes, plasma cells, heterophils, and macrophages thicken alveolar septa (up to 3 times normal thickness) and surround larger pulmonary blood vessels. Within alveolar septa and lumina, there are moderate numbers of 1 - 2 ?m, oval to fusiform basophilic organisms (tachyzoites) forming 15 - 30 ?m in diameter clusters (presumed intrahistiocytic) or, less often, arranged individually (extracellular). Multifocally, there is scattered lytic necrosis of alveolar septa characterized by disruption and replacement of septa by small amounts of necrotic cellular and karyorrhectic debris, fibrin and the aforementioned inflammatory cells. Bronchiole lumina contain refluxed edema fluid and inflammatory cells and peribronchiolar connective tissue is edematous. Alveolar septa are rarely lined by a thin layer of brightly eosinophilic fibrin (hyaline membranes).
Contributors Morphologic Diagnoses: Lung: Moderate diffuse acute interstitial pneumonia with edema, necrosis, and intralesional protozoa (etiology: Toxoplasma gondii).
Contributors Comment: Histologic findings are consistent with Toxoplasma gondii.2,3 T. gondii are apicomplexan protozoa that affect a wide range of intermediate hosts and are closely related to other coccidia including Neospora spp. and Sarcocystis spp.2
The life cycle of T. gondii includes both an intermediate and definitive host. All warm-blooded mammals, including cats and humans, can act as intermediate hosts to complete the asexual stages of the life cycle. The intermediate host is infected by ingesting the sporulated oocysts in food, water, or soil contaminated with cat feces, or less commonly, by ingesting tissue cysts in uncooked meat.1,3 Sporozoites leave the oocyst and develop into tachyzoites, which invade the lamina propria and multiply in the intestines. Tachyzoites continue to multiply first in the mesenteric lymph nodes, and then reach the rest of the body through the circulation either free or within lymphocytes, macrophages, and granulocytes. Lesions associated with acute toxoplasmosis are more commonly observed during this asexual stage with rapid multiplication of tachyzoites. The most commonly associated lesions include necrosis, edema, and inflammation (Figure 1).1-3 This case shows the lesions of an acute infection with T. gondii. In this rabbit, lesions were observed in the lung, heart, liver, spleen, kidney, adrenal gland, thyroid gland, stomach, small intestine, brain, skeletal muscle, mesenteric lymph node, and nasal turbinates. Tachyzoites can spread anywhere in the body, as evidenced in this case. Tachyzoites eventually encyst in a wide range of tissues, including brain, liver, lung, muscles, and retina. Tissue cysts may contain anywhere from two to hundreds of bradyzoites. Animals that survive the acute phase of the infection acquire immunity to T. gondii.2
Domestic and wild felids are the definitive hosts for T. gondii. Cats most commonly become infected by eating muscle containing tissue cysts. Bradyzoites are released in the gastrointestinal tract and enter the epithelial cells of the small intestines to undergo several stages of asexual and sexual multiplication. Gamonts are formed and result in oocyst formation. The prepatent period is variable and ranges from 3 18 days or more depending on the stage of the organism at the time of ingestion. Unsporulated oocysts are released into the feces where they sporulate within 24 hours in order to infect an intermediate host. Oocysts can remain viable in the environment for long periods of time.1,2
This case shows the lesions of disseminated toxoplasmosis with a rabbit as the intermediate host; the definitive host in this case is unknown. The lions may have functioned as the definitive host, or the rabbits could have acquired the infection outside of the lion enclosure. Biosecurity measures are in place to exclude feral cats and other felids from the entire facility. In general, biosecurity measures that could decrease the impact of T. gondii in zoos and other facilities include: not housing highly susceptible species (e.g. marsupials and primates) near felids, freezing meat that cannot be cooked prior to feeding animals, designing enclosures to exclude domestic cats and other vectors, and daily cleaning to prevent the sporulation of oocysts in the environment.2
JPC Diagnosis: Lung: Interstitial pneumonia, necrotizing, diffuse, moderate, with fibrin, edema, and protozoal tachyzoites (etiology consistent with Toxoplasma gondii), brush rabbit, Sylvilagus bachmani.
Conference Comment: This case nicely demonstrates the characteristic histologic lesions associated with the interstitial pneumonia associated with systemic toxoplasmosis. Conference participants described the random areas of necrosis and identified intracellular and extracellular protozoal tachyzoites within the interstitium and epithelial cells. Participants discussed the different patterns of pneumonia, interstitial, bronchopneumonia, embolic pneumonia, bronchointerstitial pneumonia, granulomatous, and uncategorizable pneumonias.
The conference moderator traditional bronchopneumonias which involves an exudate which originates at the bronchiolar-alveolar junction and fills bronchioles and alveoli . The characteristic distribution is cranioventral (due to gravity dispersal of inhaled pathgens) which is most commonly caused by opportunists . In contrast, bronchointerstitial pneumonia may be defined as either (1) bronchiolar necrosis and diffuse alveolar damage with destruction of both bronchiolar and alveolar epithelium, or (2) mononuclear cellular inflammation which surround airways and infiltrate alveolar septa. Mycoplasma hyopneumonia with its characteristic peribronchiolar and peribronchar lymphoproliferative nature was discussed as one example.4
Conference participants considered Encephalitozoon cuniculi as a potential differential for this presentation in a rabbit. Encephalitozoon cuniculi is a gram-positive, acid fast, obligate intracellular microsporidian that infects a variety of mammalian hosts, the domestic rabbit, being one of the most common. Historically, there has been disagreement among taxonomists about the classification of this organism, but genomic sequencing has confirmed it as a eukaryotic fungus within the phylum Microsporidia. Transmission occurs through ingestion or inhalation of infected urine or transplacentally. Infective spores then enter circulation via infected mononuclear cells and hit initial target organs such as lung, liver, or kidney. At approximately 3 months post infection, organisms can be found within the central nervous system and produce characteristic clinical signs (head tilt, ataxia, and vestibular signs). In dwarf rabbits, phaecoclastic uveitis and cataract formation are quite common after transplacental transmission. In the lung, lesions may appear as a focal to diffuse interstitial pneumonia with mononuclear cellular infiltration. Microscopically, E. cuniculi and T. gondii can look quite similar; staining characteristics can help differentiate the two. Toxoplasma organisms are Gram-negative and do not stain with carbol fuschin stains (a type of acid fast stain, a Ziehl-Neelsen subcomponent stain).2 Although, the contributor prudently identified toxoplasmosis using PCR, Gram stains and several acid fast stains (Ziehl-Neelsen and Fite-Faraco; we do not have access to Carbol fuschin) were performed to further rule out encephalitozoonosis. Organisms within submitted sections were strongly Gram negative, and were not highlighted with acid fast stains which confirm the contributors diagnosis of Toxoplasma gondii.
Finally, conference participants discussed that Toxoplasmosis has a military and veterinary public health relevance due to its recent identification in the central nervous system of individuals suffering from various mental health disorders (bipolar disorder, post-traumatic stress disorder, schizophrenia) and related suicides.1
|
Toxoplasma |
Encephalitozoon |
|
Small cyst 60 |
Large pseudocyst up to 120 |
|
Spores not acid fast |
Spores are acid fast |
|
Gram negative |
Gram positive |
|
Do not stain with carbol fuchsin |
Stain with carbol fuchsin (purple) |
|
Giemsa: granulated cytoplasm |
Giemsa: light blue cytoplasm |
|
Stains well with H&E |
Stains poorly with H&E |
|
Larger organism 2-6 um |
Smaller organism 1.5 x 2.5 um |
|
Tend to invoke necrosis |
Necrosis is not a feature |
Contributing Institution:
Wildlife Disease Laboratories
Institute for Conservation Research
San Diego Zoo Global
http://www.sandiegozooglobal.org
References:
1. Ansari-Lari M, Farashbandi H, Mohammadi F. Association of Toxoplasma gondii infection with schizophrenia and its relationship with suicide attempts in these patients. Trop Med Int Health. 2017; 22:epub ahead of print. doi: 10.1111/tmi.12933.
2. Barthold SW, Griffey SM, Percy DM. Rabbit. In: Pathology of Laboratory Rodents and Rabbits. 4th ed. Oxford, UK: John Wiley & Sons, Inc.; 2016:293-295.
3. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 2. 5th ed. Edinburgh, UK: Elsevier Limited; 2007:270-272.
4. Caswell JL, Williams KJ. Respiratory system. In: Maxie MG, ed. Jubb, Kennedy and Palmers Pathology of Domestic Animals. Vol 2. 6th ed. Edinburgh, UK: Elsevier Limited; 2016:506-511.
5. Dubey JP, Odening K. Toxoplasma and related infections. In: WM Samuel, MJ Pybus, and AA Kocan, ed. Parasitic Diseases of Wild Mammals, 2nd ed. Ames, IA: Iowa State University Press; 2001: 478-492.
6. Gardiner CH, Fayer R, Dubey JP. An Atlas of Protozoan Parasites in Animal Tissues, 2nd edition. Armed Forces Institute of Pathology. Washington, DC. 1998.