Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 16
29 January 2020
Dr. Ingeborg
Langohr, DVM, PhD, DACVP
Professor
Department of Pathobiological Sciences
Louisiana State University School of Veterinary Medicine
Baton Rouge, LA
CASE II: 2015A (JPC 4167277).
Signalment: Four-day-old crossbreed piglet (Sus scrofa domesticus)
History: This pig had no previous signs of illness, and was found dead.
Gross Pathology: At necropsy, the stomach was filled with undigested curdled milk. The small and large intestines were distended by watery yellow-to-greenish undigested milk, and intestinal walls were thin. No gross lesions were detected in other organs.
Laboratory results: RT-PCR for porcine epidemic diarrhea (PED) virus (PEDV) and transmissible gastroenteritis (TGE) virus (TGEV) was performed, the expected size of amplification products for PEDV gene, but not TGEV gene, were obtained. The RT-PCR products were directly sequenced and confirmed as PEDV gene.
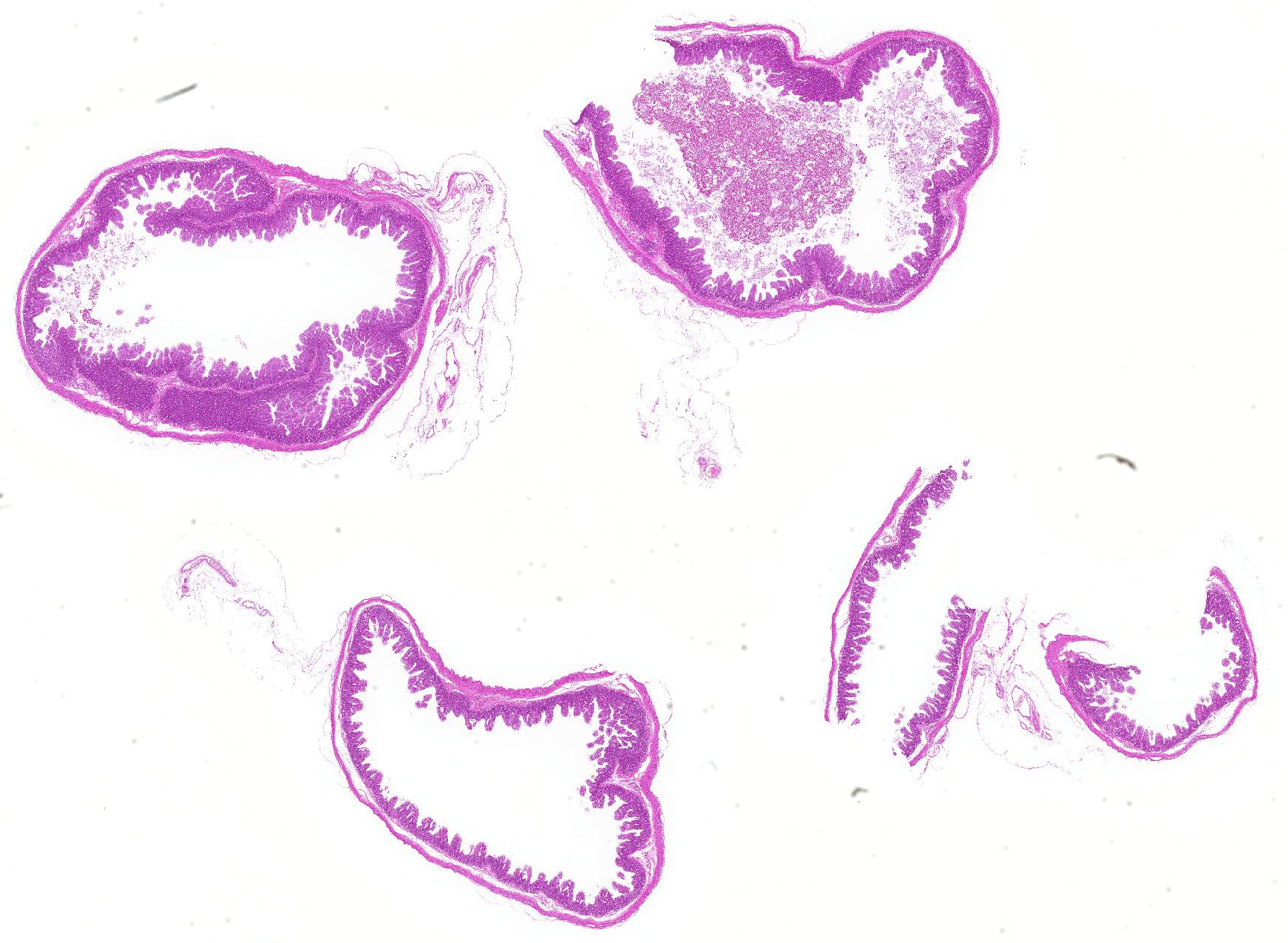
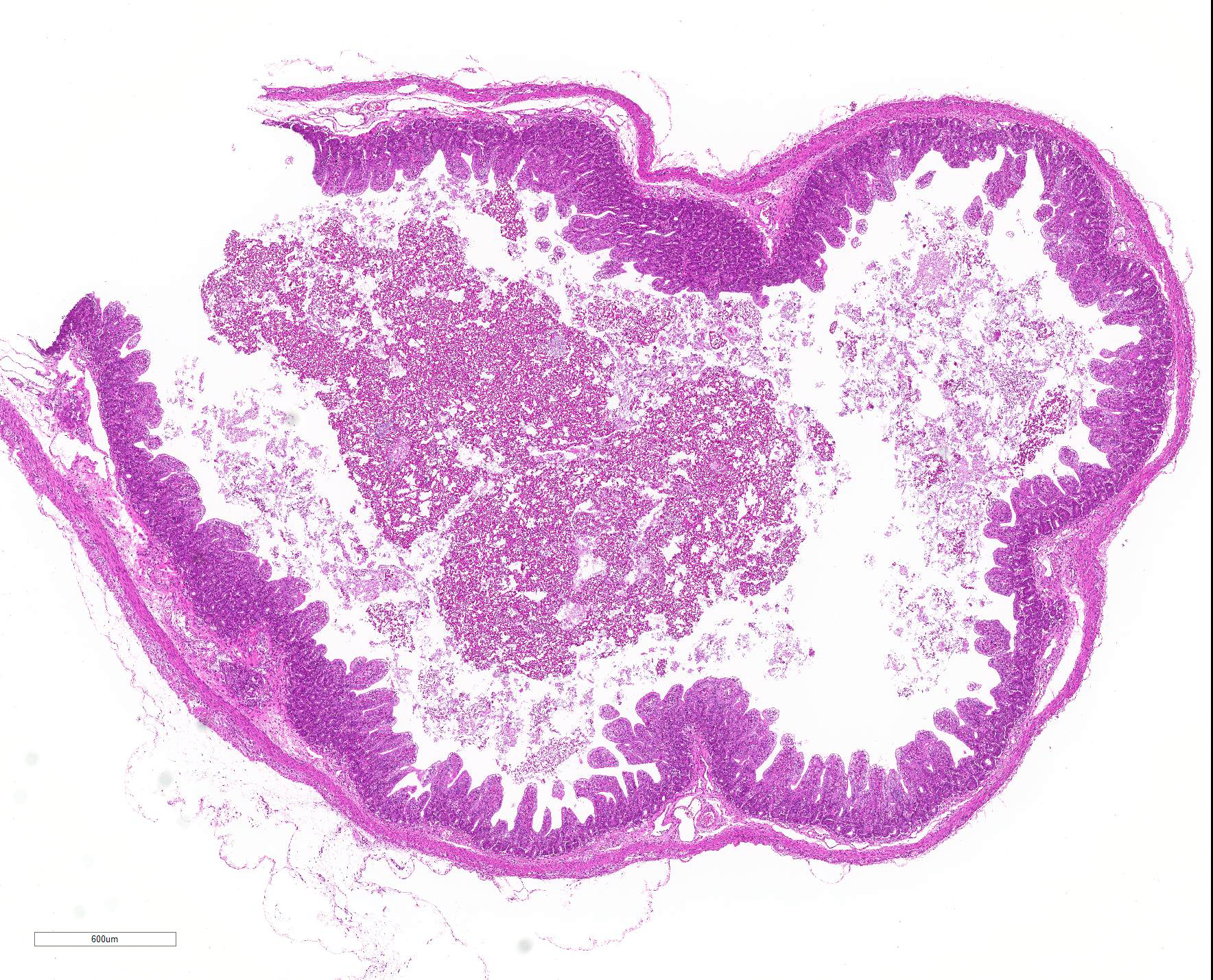
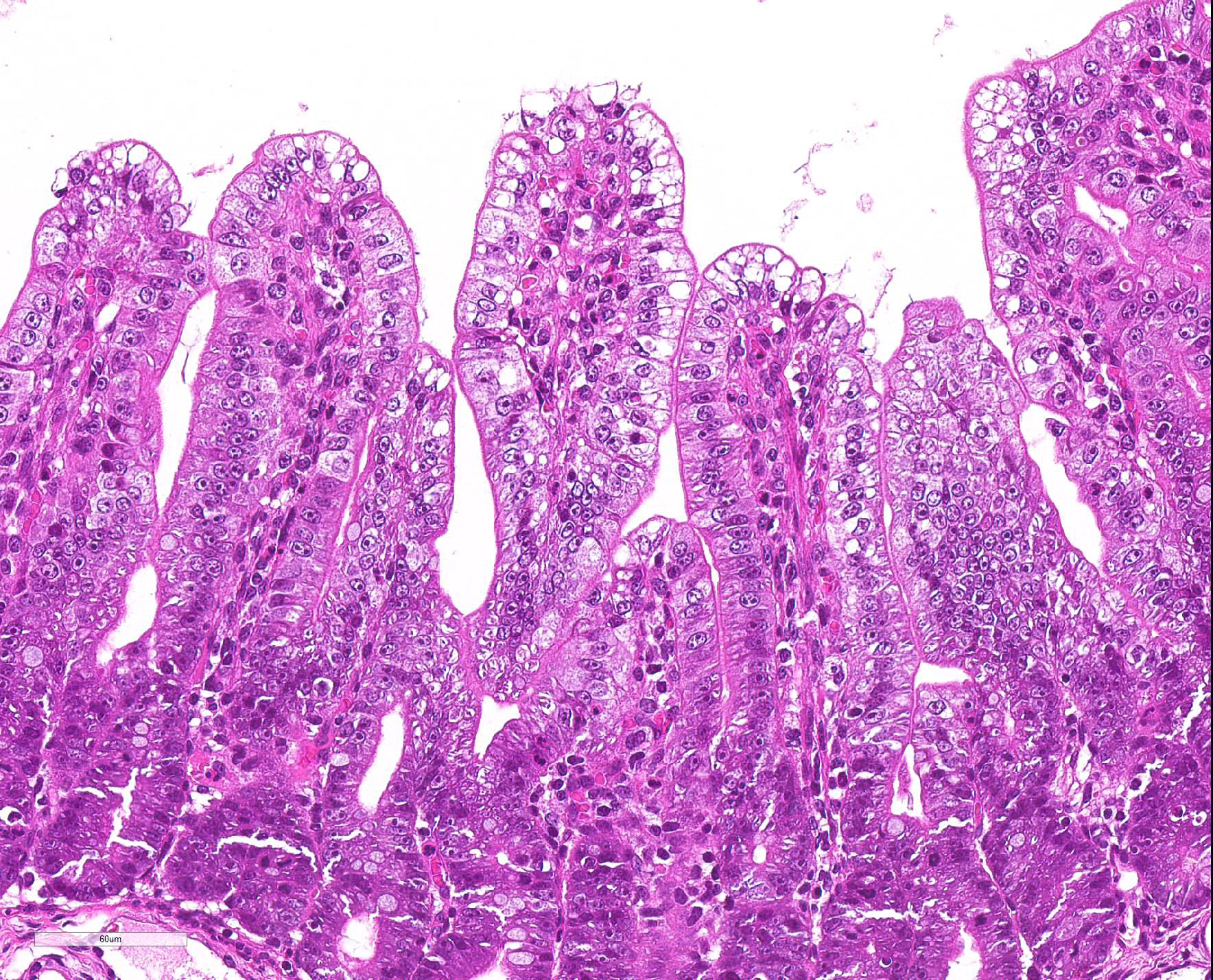
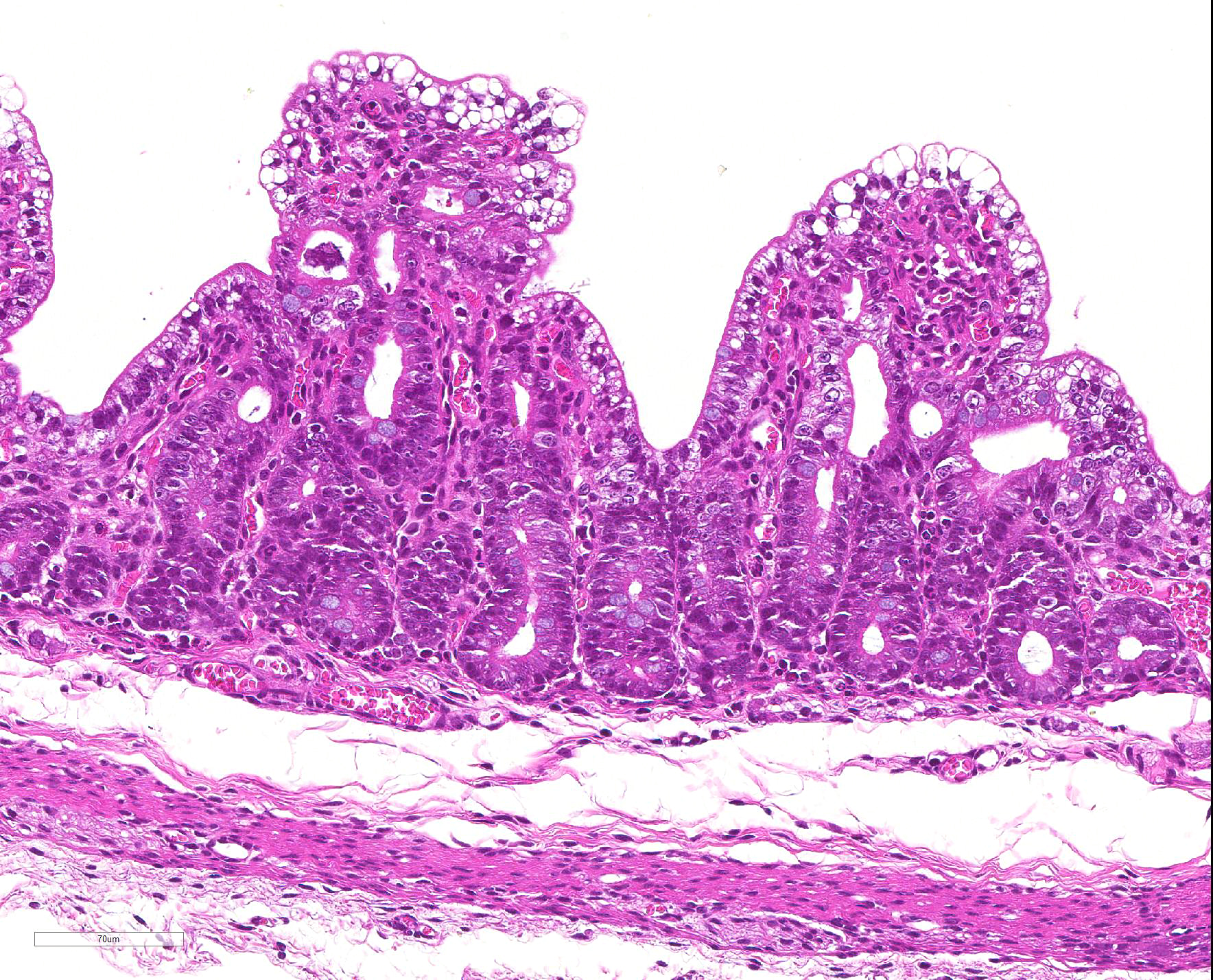
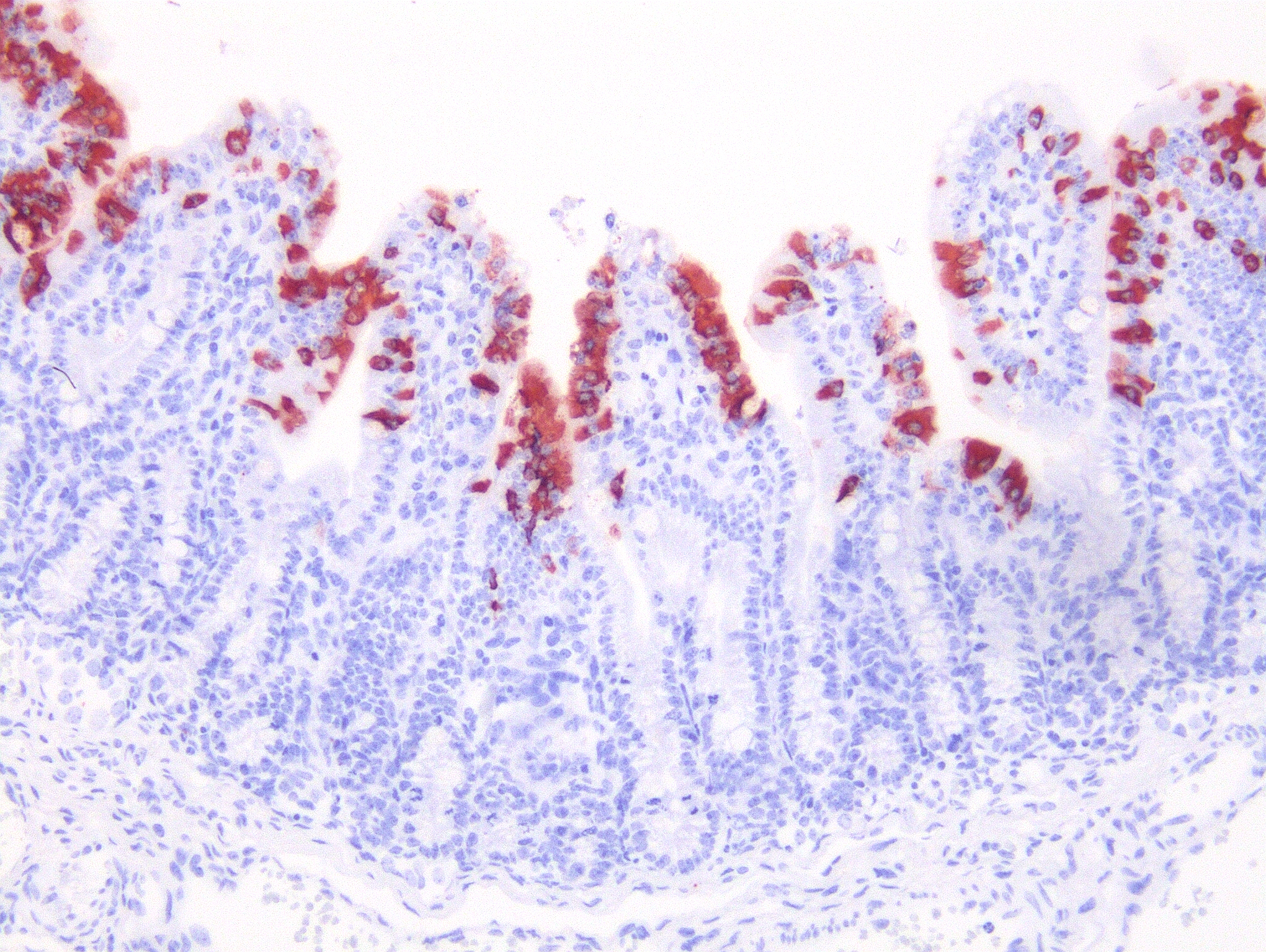
Microscopic Description: Severe atrophy of villi was detected in all segments of the small intestine. There were vacuolated epithelial cells throughout the small intestine. Immunohistochemical analysis revealed presence of PED viral antigen in the cytoplasm of epithelial cells lining the jejunum. No histopathologic lesions were detected in other organs.
Contributor Morphologic Diagnosis:
Jejunum: Enteritis, villous atrophy, acute, diffuse, severe.
Contributor Comment: PEDV is an enveloped, positive-sense, single-stranded RNA virus that belongs to the order Nidovirales, family Coronaviridae, genus Alphacoronavirus. PED was first observed among English feeding and fattening pigs in 1971, and then emerged in many European, Asian and North American countries.9 PED is a highly contagious disease of swine of all ages, and has become a devastating issue in many pig-raising countries in Asia and North America.1,4,5,7 In October 2013, a PED outbreak was recurred and confirmed in Japan after an absence of seven years in the country.3 38 out of 47 prefectures are affected until August 2014, and 817 have been affected among 5,570 farms.3 PEDV isolates from this outbreak are genetically related to the PEDV isolates recovered from China and the USA in 2013.3 Several new variants of PEDV emerged in the global pig population.1,4,5,7 The spike protein of PEDV plays pivotal roles in viral entry and inducing the neutralizing antibodies in natural hosts.6 Attenuated live vaccines using cell-culture-adapted PEDVs have long been used in Asia for the control of PEDV.6,8 Recently, it was reported that an inactivated vaccine made from a U.S. field isolate is immunogenic in pigs.2
PED is characterized by vomiting and watery diarrhea, followed by dehydration, and a high mortality among suckling pigs.5,9 Differential diagnoses for vomiting and diarrhea in pigs include PED and TGE, both of which have indistinct gross and histologic lesions centered mainly on the jejunum and ileum. The diffuse villus atrophy of the small intestine is characteristic of the two disorders.9 The presence of PED virus is confirmed by immunohistochemistry and RT-PCR.9
Contributing Institution:
National Institute of Animal Health, Japan.
http://www.naro.affrc.go.jp/org/niah/
JPC Diagnosis: Intestine: Villar blunting, diffuse, severe, with villar enterocyte vacuolar degeneration, villar fusion, and mild crypt hyperplasia.
JPC Comment: Porcine epidemic virus (PEDV) outbreaks were common in Europe in the 1970s and 1980s, and in Asia in the 1980s to 2000s. The first outbreak of PEDV in the United States occurred in April 2013 with explosive epidemics of diarrhea and vomiting affecting all ages, and resulting in 90-95% mortality in suckling pigs.9
In
affected pigs, the main and often only sign of disease is watery diarrhea
affecting up to 100% of pigs in all age groups, although piglets are more
commonly affected.. Experimental studies have demonstrated a 22- to 36-hour
incubation period with viral replication in villous epithelium in all segments
of the small intestine (viral replication can also been seen in colonic
epithelium, but without obvious cellular degeneration).9 Villus:crypt
ratios in affected pigs are decreased from 7:1 to 3:1 or less. Characteristic
vacuolar change of the villous epithelium resemble that seen with the
coronavirus that causes transmissible gastroenteritis (TGE) in pigs, but are
considered somewhat less pronounced.9
Clinical signs of disease on farms with naïve animals mimic those of epidemic transmissible
gastroenteritis virus (TEGV) infection. Mortality is highest in piglets of 1
week of age or less, which often die of dehydration after 3-4 days of illness.
Older pigs recover after about a week although recurrent diarrhea may occur in
times of stress during intestinal repair, and sows may exhibit depression but
no gastrointestinal signs.10 The disease in the breeding facility
is self-limiting, and tends to disappear sows develop immunity and can produce colostral
antibodies.10
Other coronaviruses of interest, but of less repute can also cause clinical disease in swine. A betacoronavirus known as hemagglutinating encephalomyelitis virus results in a disease known as vomiting and wasting disease. Infection is consided widespread among swine, although clinical disease is incommon. The virus, as the name suggests, possesses the ability to spontaneous agglutinate the erythrocytes of a number of species, including laboratory rodents.10 The virus infects the respiratory epithelium of pigs less than 4 weeks of age, which lack protective antibodies. The virus ascends to the CNS via trigeminal, vagal, or spinal nerves. Vomiting in affected pigs is triggered by replication in the vagal sensory ganglion or in nerves that terminate in the vomiting center. Prolonged vomiting results in wasting; young piglets may die of dehydration, older pigs become emaciated.10
Another lesser known member of the Coronaviridae family is porcine torovirus, one of three species of torovirus, all of which rarely cause clinical disease. In diarrheic pigs, seroprevalence for porcine torovirus may be high in many countries; however, concomitant enteric pathogens are simultaneously identifed in approximately 75% of cases so a direct link between porcine torovirus and true enteric disease is difficult to prove.10
The moderator mentioned the normal crypt ratio of 7:1 for a normal piglet, which demonstrates the marked villar blunting in this individual. A pathologist in the audience comment on the thin muscular tunics; the moderator noted that the muscular tunics of the intestine are thickest in proximity of the gastroduodenal junction and diminish caudally.
The moderator mentioned a number of other coronaviruses causing enteric disease in pigs including the porcine deltacoronavirus, and a bat-origin alphacoronavirus which resulting in almost 25,000 dead piglets on 4 farms in China in October 2017.
References:
1. Chen Q, Li G, Stasko J, Thomas JT, Stensland WR, Pillatzki AE, Gauger PC, Schwartz KJ, Madson D, Yoon KJ, Stevenson GW, Burrough ER, Harmon KM, Main RG, Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol. 2014;52(1):234?243.
2. Collin EA, Anbalagan S, Okda F, Batman R, Nelson E, Hause BM. An inactivated vaccine made from a U.S. field isolate of porcine epidemic disease virus is immunogenic in pigs as demonstrated by a dose-titration. BMC Vet Res. 2015;11(1):62.
3. European Food Safety Authority (EFSA), Parma, Italy. Scientific Opinion on porcine epidemic diarrhoea and emerging porcine deltacoronavirus. EFSA Panel on Animal Health and Welfare (AHAW). EFSA J. 2014;12(10):3877.
4. Huang YW1, Dickerman AW, Piñeyro P, Li L, Fang L, Kiehne R, Opriessnig T, Meng XJ. Origin, evolution, and genotyping of emergent porcine epidemic diarrhea virus strains in the united states. mBio. 2013;4(5):e00737?13.
5. Lee S, Lee C. Outbreak-related porcine epidemic diarrhea virus strains similar to US strains, South Korea, 2013. Emerg Infect Dis. 2014;20(7):1223-1226.
6. Sato T, Takeyama N, Katsumata A, Tuchiya K, Kodama T, Kusanagi K. Mutations in the spike gene of porcine epidemic diarrhea virus associated with growth adaptation in vitro and attenuation of virulence in vivo. Virus Genes. 2011;43(1):72-78.
7. Song D, Huang D, Peng Q, Huang T, Chen Y, Zhang T, Nie X, He H, Wang P, Liu Q, Tang Y. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea viruses associated with outbreaks of severe diarrhea in piglets in jiangxi, china 2013. PLoS One. 2015;10(3):e0120310.
8. Song DS, Oh JS, Kang BK, Yang JS, Moon HJ, Yoo HS, Jang YS, Park BK. Oral efficacy of Vero cell attenuated porcine epidemic diarrhea virus DR13 strain. Res Vet Sci. 2007;82(1):134-140.
9. Stevenson GW, Hoang H, Schwartz KJ, Burrough ER, Sun D, Madson D, Cooper VL, Pillatzki A, Gauger P, Schmitt BJ, Koster LG, Killian ML, Yoon KJ. Emergence of Porcine epidemic diarrhea virus in the United States: clinical signs, lesions, and viral genomic sequences. J Vet Diagn Invest. 2013;25(5):649-654.
10. Zimmerman JJ, Benfield DA, Dee SA, Murtaugh MP, Stadejek T, Stevenson GW, Torremorrell M. Coronaviridae. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW, eds. Diseases of Swine. 10th ed. Ames, IA: Blackwell Publishing; 2012: 514-521.