Signalment:
Gross Description:
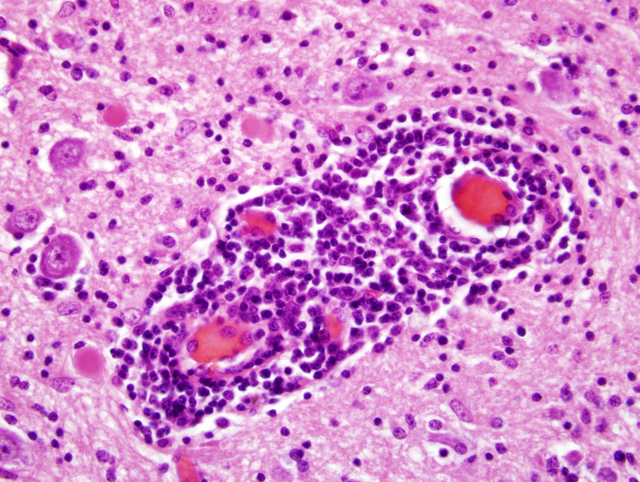
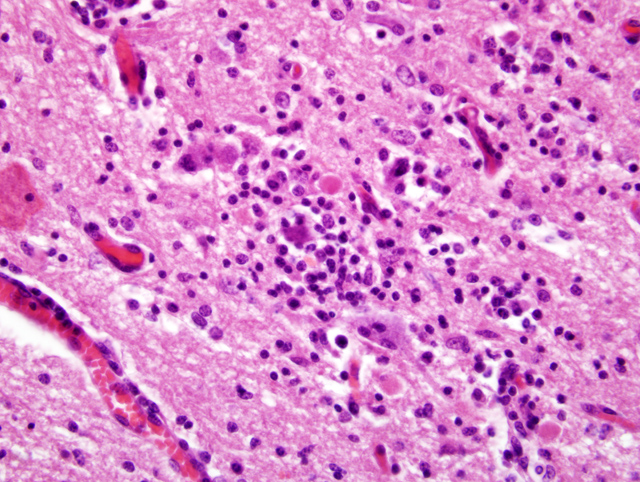
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
The highly neurotropic but noncytolytic virus (9), similar to rabies virus, is transported by retrograde axonal transport from the periphery to the CNS. After infection, BDV causes a persistent infection of the central nervous system and induces an immune-mediated encephalomyelitis. The infiltrating immune cells have been characterized as CD4- positive T-cells, CD8-positive T-cells, macrophages and B cells.(11) The virus can then also spread centrifugally via the peripheral nervous system, resulting in infection of nonneural tissue.(9) A proposed mechanism for the behavioral changes involves protein interference with neurotransmitter function of infected neurons, especially those located in the limbic lobe.(12)
The first evidence that some human psychiatric patients might be infected with BDV, or a BDV-related virus, came from serological studies using immunofluorescence assays.(13) However, several key experiments could not be reproduced by independent laboratories and started a still ongoing, highly controversial, worldwide debate about whether BDV infects humans and causes psychiatric problems.(1,14,15) In this case, the detection of viral antigen in fresh brain tissue by immunohistochemistry, the clinical manifestations and the characteristic histopathological changes within the brain all contribute to the diagnosis of Borna disease.
JPC Diagnosis:
Conference Comment:
Gross lesions are not seen in infections with BDV. Histologic lesions are generally present in the grey matter of the olfactory bulbs, hippocampus, limbic system, basal ganglia, and brain stem. The cerebellum and dorsal aspect of the cerebrum are usually unaffected.(10) Common histologic lesions are a nonsuppurative encephalitis with perivascular cuffing composed of mononuclear cells, gliosis, and neuronophagia. Perivascular cuffing is often pronounced with cells layering upon one another up to seven cells deep.(10) Inclusion bodies (Joest-Degen bodies) are nearly pathognomonic for BDV infection, and, if present, are normally in nuclei and rarely in the cytoplasm. The hippocampus is reported as the best area to find inclusions.(10) This virus is also unique because it replicates in the nucleolus of the host cell.(10)
Dr. Van Winkle discussed the importance of narrowing this particular case down to a list of differentials, and then using the tools available at your particular institution to make a diagnosis. In horses, differentials for this lesion include: West Nile virus, Japanese encephalitis virus, Murray Valley encephalitis, St. Louis encephalitis, Western equine encephalitis, Eastern equine encephalitis, and Venezuelan equine encephalitis.(1) Other potential differentials discussed during the conference included equine protozoal myelitis and equine herpesvirus type 1. Dr. Van Winkle commented that geographic location in many cases of equine encephalitis is a good indicator of what differential should be at the top of the list.Â
References:
2. Carbone KM: Borna disease virus and human disease. Clin Microbiol Rev 14:513-527, 2001
3. Carbone KM, Duchala CS, Griffin JW, Kincaid AL, Narayan O: Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J Virol 61:3431-3440, 1987
4. Carbone KM, Rubin SA, Nishino Y, Pletnikov MV: Borna disease: virus-induced neurobehavioral disease pathogenesis. Curr Opin Microbiol 4:467-475, 2001
5. de la Torre JC, Carbone KM, Lipkin WI: Molecular characterization of the Borna disease agent. Virology 179:853-856, 1990
6. Hagiwara K, Okamoto M, Kamitani W, Takamura S, Taniyama H, Tsunoda N, Tanaka H, Iwai H, Ikuta K: Nosological study of Borna disease virus infection in race horses. Vet Microbiol 84:367-374, 2002
7. Honkavuori KS, Shivaprasad HL, Williams BL, Quan PL, Hornig M, Street C, Palacios G, Hutchison SK, Franca M, Egholm M, Briese T, Lipkin WI: Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg Inf Dis Vol 14, Num 12, Dec 2008
8. Inoue Y, Yamaguchi K, Sawada T, Rivero JC, Horii Y: Demonstration of continuously seropositive population against Borna disease virus in Misaki feral horses, a Japanese strain: a four-year follow-up study from 1998 to 2001. J Vet Med Sci 64:445-448, 2002
9. Kamhieh S, Flower RL: Borna disease virus (BDV) infection in cats. A concise review based on current knowledge. Vet Q 28:66-73, 2006
10. Maxie MG, Youssef S: Nervous system. In: Jubb, Kennedy and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol 1, pp.421-426. Elsevier Limited, Philadelphia, PA, 2007
11. Morales JA, Herzog S, Kompter C, Frese K, Rott R: Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med Microbiol Immunol 177:51-68, 1988
12. Planz O, Bilzer T, Stitz L: Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J Virol 69:896-903, 1995
13. Reeves NA, Helps CR, Gunn-Moore DA, Blundell C, Finnemore PL, Pearson GR, Harbour DA: Natural Borna disease virus infection in cats in the United Kingdom. Vet Rec 143:523-526, 1998
14. Rott R, Herzog S, Fleischer B, Winokur A, Amsterdam J, Dyson W, Koprowski H: Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science 228:755-756, 1985
15. Staeheli P, Sauder C, Hausmann J, Ehrensperger F, Schwemmle M: Epidemiology of Borna disease virus. J Gen Virol 81:2123-2135, 2000
16. Weissenbock H, Nowotny N, Caplazi P, Kolodziejek J, Ehrensperger F: Borna disease in a dog with lethal meningoencephalitis. J Clin Microbiol 36:2127-2130,1998