WSC 2022-2023:
Conference 16:
Case II:
Signalment:
Juvenile, male intact, bobcat (Lynx rufus)
History:
The bobcat presented to a wildlife rehabilitation center for right hind lameness, dehydration and emaciation. The bobcat initially improved with therapy but after a couple of weeks of hospitalization deteriorated rapidly and died.
Gross Pathology:
The spleen was slightly enlarged with a meaty consistency and had multifocal, sparse, pinpoint, white foci throughout the parenchyma. The retropharyngeal, mesenteric and renal lymph nodes were moderately to markedly enlarged and on the cut surface had many white to pale tan foci. The liver was brown and had multifocal, pinpoint to 0.1 cm white foci throughout the parenchyma. The right distal tibia was slightly expanded by a bony proliferation (interpreted as callus). The abdominal cavity contained approximately 40 ml of yellow, transparent, viscous to slightly gelatinous fluid.
Laboratory Results:
Immunohistochemistry:
Feline coronavirus antigen specific immunohistochemistry performed on the section of ileum was negative.
Bacteriology:
Francisella tularensis was isolated from the liver by aerobic culture.
Molecular diagnostics:
The isolated bacteria were confirmed to be Francisella tularensis type A based on PCR.
Microscopic Description:
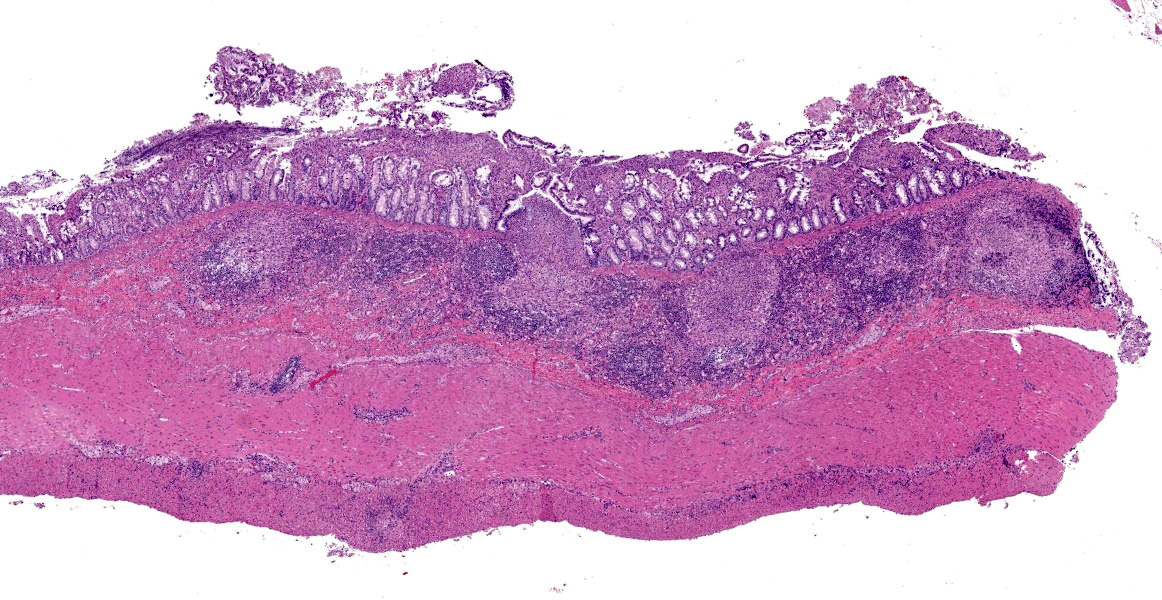
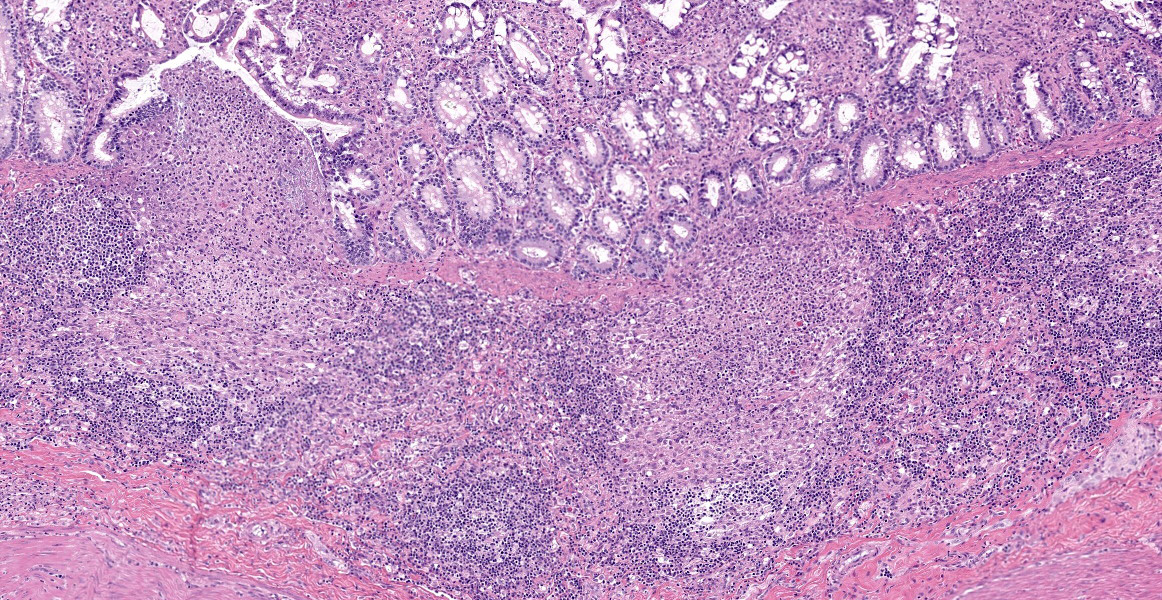
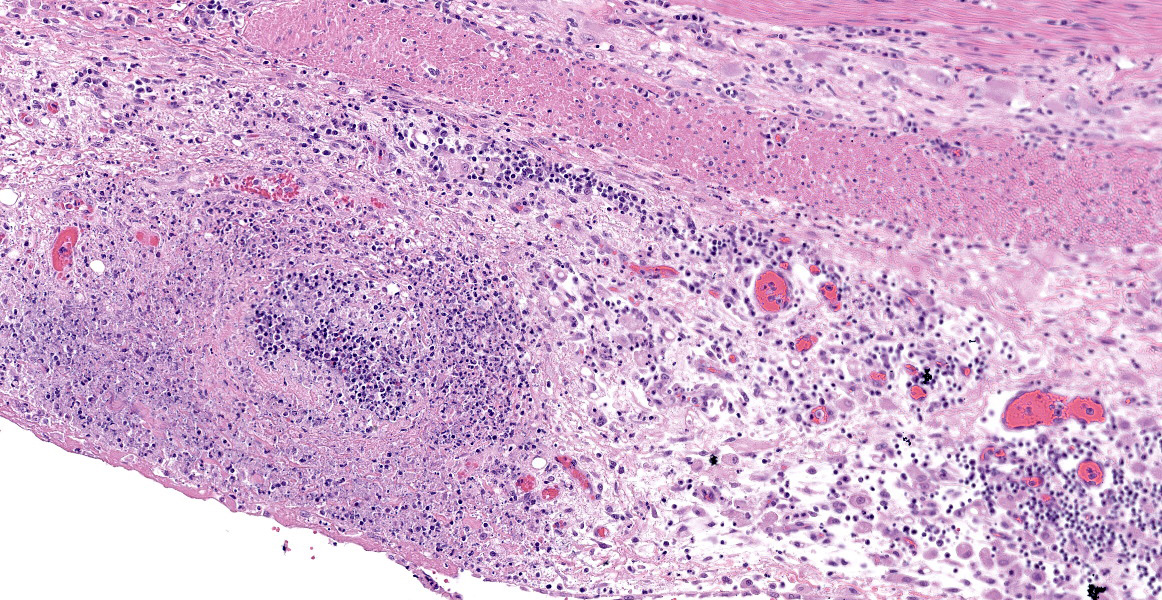
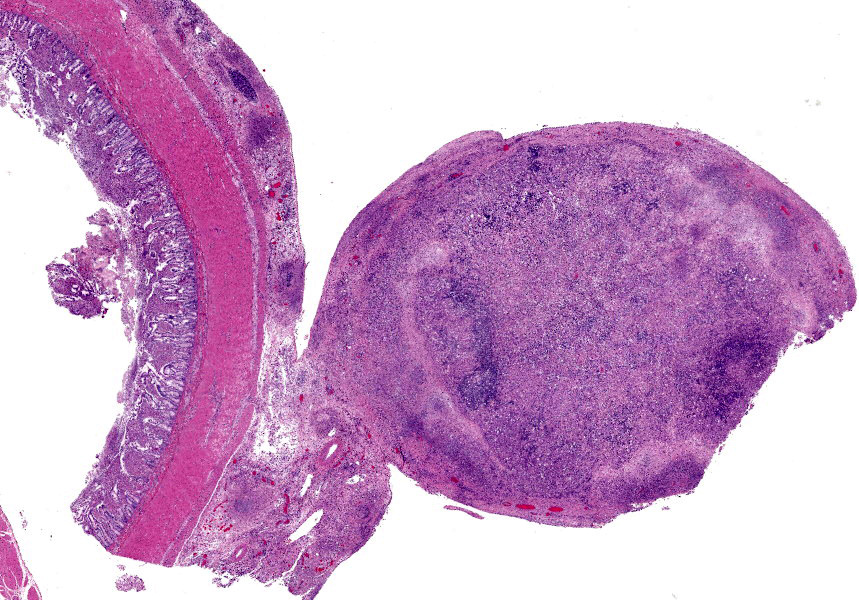
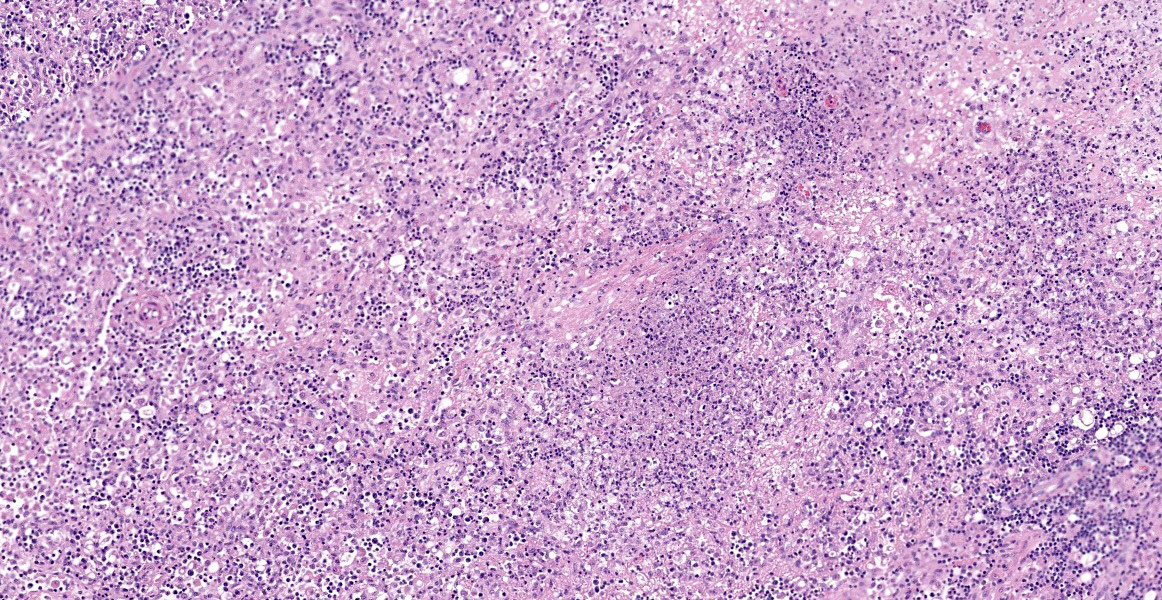
Ileum - Multifocally, Peyer’s patches are largely effaced by variably-sized, central areas of eosinophilic cellular and basophilic karyorrhectic debris admixed with degenerate neutrophils (lytic necrosis) which are surrounded by moderate to large numbers of epithelioid macrophages. Multifocally infiltrating the surrounding submucosa are moderate numbers of lymphocytes, plasma cells, neutrophils and macrophages. The outer longitudinal lamina muscularis is multifocally effaced by small areas of lytic necrosis and cellular debris, and within the inner circular lamina muscularis there are multifocal, often perivascular clusters of lymphocytes, plasma cells, neutrophils and macrophages. The attached mesentery is multifocally effaced by large areas of lytic necrosis as well as moderate numbers of lymphocytes, plasma cells and macrophages with fewer neutrophils, admixed with fibrillar, brightly eosinophilic material (fibrin). Within the mucosa there is a reduced density of crypts, with widespread blunting of villi and expansion of the lamina propria with moderate numbers of neutrophils and fewer lymphocytes, plasma cells and macrophages. Within the superficial mucosa and intestinal lumen are multifocal small colonies of mixed bacteria, as well as moderate numbers of apicomplexan coccidian organisms in various developmental stages, including: few shizonts containing numerous basophilic, elongate merozoites; numerous uninucleate, eosinophilic macrogametes; numerous oocysts containing pale eosinophilic granular material; and rare large microgamonts with numerous condensed, basophilic nuclei.
Mesenteric lymph node - The mesenteric lymph node associated with the ileum is markedly expanded and almost completely effaced by a large, central area of lytic necrosis as previously described, admixed with macrophages, neutrophils and fibrin. Few small to moderately-sized aggregates of mature lymphocytes and plasma cells remain scattered throughout the section.
Contributor’s Morphologic Diagnoses:
1. Ileum:
a. Ileitis, pyogranulomatous and necrotizing, multifocal, severe, chronic.
b. Intraepithelial coccidian organisms, moderate.
2. Mesenteric lymph node, lymphadenitis, pyogranulomatous and necrotizing, widespread, severe, chronic.
Contributor’s Comment:
Francisella tularensis, the causative agent of tularemia, is a gram negative, obligate aerobe, intracellular coccobacillus of the gamma-subclass of Proteobacteria.10,12 There are currently 4 recognized subspecies: subspecies tularensis (type A), which is highly virulent in domestic animals and humans, predominantly found in North America, and is most commonly associated with lagomorphs; subspecies holarctica (type B), which is less virulent, endemic throughout the Northern Hemisphere, and more frequently associated with rodents and water-borne disease; subspecies novicida, which is mostly avirulent in humans and predominantly found in North America; and subspecies mediasiatica, which is also considered to be avirulent in humans and predominantly found in Russia and central Asia.11 The type A subspecies is further divided into 2 distinct subpopulations, A1 and A2. In the USA, A1 subpopulations occur primarily in the eastern half of the country while A2 subpopulations occur primarily in the west.9
F. tularensis has a wide and varied range of hosts, with a list of known susceptible species including 190 mammals, 88 invertebrates, 23 birds and 3 amphibians, with occasional reports in reptile and fish species.6 Although lagomorphs and rodents are frequently implicated in the spread and maintenance of this disease, there has been no evidence to suggest that they act as a major reservoir.7,12 Transmission to humans can occur via a number of different routes, including handling of infected animals, ingestion of improperly cooked infected meat or contaminated water, inhalation of infective aerosols, and arthropod bites.9 Important arthropod vectors for transmitting F. tularensis in the USA include hard ticks, deer flies, stable flies and horseflies. Of those, hard ticks are considered to be the primary vectors for type A strains; ticks of the Ambylomma and Dermacentor genera are typically responsible for human cases while ticks of the Ixodes and Haemophysallis genera are more important in maintaining enzootic foci among wildlife.4,9 Sheep are historically considered to be the domestic mammal most commonly associated with tularemia type A, however, in the past few decades domestic cats have become an increasing source for zoonotic transmission.4
Gross lesions in affected animals typically involve miliary white foci within the spleen, liver and lymph nodes, as well as splenomegaly, hepatomegaly and lymphadenopathy.8 These lesions are grossly indistinguishable from the lesions caused by Yersinia species. Histologically, these white foci are composed of multifocal areas of lytic necrosis, admixed with degenerate neutrophils and/or surrounded by macrophages and fibroblasts in older lesions. F. tularensis bacteria are generally difficult to visualize histologically in routine H&E and even Gram stained sections, whereas Yersinia species bacteria tend to be readily visible.
In this bobcat, there was a translucent, straw colored, viscous, serofibrinous abdominal effusion, reminiscent of the effusion which characterizes the wet form of feline infectious peritonitis (FIP). In the submitter’s experience, this is an atypical finding for tularemia. Therefore, and because bacteria were not detected within the lesions, immunohistochemistry for feline coronavirus antigen was performed on the section of ileum; however, coronaviral antigen was not detected within any of the cells in the section.
The coccidian organisms within the lumen of this bobcat’s ileum were morphologically most similar to Cystoisospora felis. In a recent paper, C. felis-like organisms were found in feces of 2 bobcats and transmitted to domestic cats.2 Unlike C. felis in domestic cats which develops within the villar epithelium, the schizonts and gamonts of these C. felis-like coccidia were located within the lamina propria of the ileum, indicating that they are likely to be a similar but different parasite to C. felis of domestic cats. In our bobcat’s case, it is difficult to assess the organisms’ location with certainty due to the significant blunting and disruption of the villi. In addition, the significance of the mixed colonies of bacteria within the intestinal lumen is unknown. While they may have contributed to the suppurative ileitis, it is also possible that they represent an intraluminal ‘bystander’ population.
F. tularensis is a tier 1 agent on the US Department of Health and Human services biological select agents list as it has the potential to pose a severe threat to public health and safety.3 As a consequence of being on this list, there are strict regulations involved when handling F. tularensis. The agent and all tissues that contain the agent must be destroyed, and this destruction must be reported. Appropriate personal protective equipment (PPE) should be worn when this agent is suspected, including gloves and a cut-resistant glove, an impermeable apron, lab boots, a face shield, and a respirator such as a properly fitted N95 mask.
Contributing Institution:
Veterinary Diagnostic Laboratory, University of Minnesota, St Paul, MN.
https://vdl.umn.edu/
JPC Diagnosis:
- Ileum: Ileitis, necrotizing, diffuse, moderate, with multifocal vasculitis and Peyer’s patch necrosis.
- Mesenteric lymph node: Lymphadenitis, necrotizing, diffuse, severe.
- Mesentery, vessels: Vasculitis, necrotizing, severe.
- Ileum: Coccidial gamonts and oocysts, multiple.
- Pancreas and adipose tissue: Zymogen granule depletion and fat atrophy.
JPC Comment:
During the conference, the participants and moderator, COL(R) Derron (Tony) Alves, remarked on the marked vasculitis along the serosa and within the mesentery and prominent reactive mesothelium suggestive of abdominal effusion. Before knowing the clinical history and laboratory results, participants considered the possibility of FIP based on these histologic lesions, but the contributor ruled this out using immunohistochemical staining. Participants also had spirited discussion regarding inflammation surrounding areas of lymphoid necrosis in Peyer’s patches and lymph nodes; some felt this would qualify as pyogranulomatous inflammation, while others felt that the remaining cells represented residual dendritic cells associated with lymphoid follicles.
In 1909, a researcher investigating bubonic plague in squirrels in California discovered a novel bacterium which caused enlarged lymph nodes.5 The bacteria was dubbed Bacterium tularense, reflecting where it was discovered - Tulare county. This new bacterium was not directly contagious but could be transmitted via infected blood, tissue, or fleas. 5 The first confirmed infection in a human occurred in a butcher in Ohio in 1911. 5 In the early 1920s, researchers in Montana conducting wood tick surveillance for Rocky Mountain Spotted Fever discovered that some ticks were infected with B. tularense and could pass this infection to animals. 5 Furthermore, these ticks could transmit the bacterium transovarially and would remain infected for life. 5
Around the same time, Edward Francis began his research in Utah on “Deer Fly Fever”, a condition in humans caused by B. tularense featuring fever, lymphadenitis, and draining tracts from affected nodes.5 In 1928, Francis published a thorough report on 629 human cases of B. tularense, and the bacterium was later renamed Francisella tularense in acknowledgement of his extensive research.5 This rechristening was also fitting in that Francis was frequently affected by the disease himself: he was infected at least five times during his life, likely due to his reluctance to wear gloves during autopsies.5
Of the cases reported by Francis, 400 were attributed to contact with rabbits, and 70 were attributed to tick bites.5 The disease had an incubation period of less than 10 days and resulted in acute fever with two to three months of convalescence.5 The most common clinical presentation was the ulceroglandular form, as an ulcer formed at the site of exposure and regional lymphadenitis resulted in a draining tract over the inflamed “gland”.5 Other reported forms included oculoglandular (where conjunctival exposure resulted in conjunctivitis, corneal ulceration, and regional lymphadenitis), glandular form (similar to ulceroglandular form without cutaneous lesions), and typhoidal (systemic disease lacking ocular, cutaneous, and lymph node involvement).5 Additional forms subsequently described include an oropharyngeal form and pneumonia.5
F. tularensis is a facultative intracellular parasite which can survive and replicate in a variety of host cells, including macrophages, dendritic cells, neutrophils, endothelial cells, type II pneumocytes, and hepatocytes.1 The pathogencity of Francisella is associated with intracytoplasmic replication within macrophages.13 The bacteria induces phagocytosis by binding to mannose receptors; the C3 complement receptor, scavenger receptor A, nucleolin, or lung surfactant protein A once opsonized; or the Fcγ receptor once antibody-opsonized.1 The microbe survives within the phagosome by delaying phagosome maturation, inhibiting NADPH oxidase activation, and resisting the effects of reactive oxygen species.1,13 While other bacteria which live in the cytosol use lipid hydrolases or phospholipases to escape the phagosome (such as Listeria), Francisella is unique in that it likely uses a type VI secretion system for phagosome egress.1,13 The bacterium then uses “nutritional virulence” to adapt to the cytosolic environment.13 Francisella LPS has low endotoxicity and is not recognized by TLR4; rather, the bacteria triggers TLR2 signaling, activates both the AIM-2 and NLRP3 inflammasomes, and ultimately results in production of proinflammatory cytokines IL1 beta and pro-IL8.1 Apoptosis or pyroptosis of infected cells leads to liberation of the bacteria, which can then infect new cells.1,13 The bacteria can also directly penetrate adjacent cells using trogocytosis.13
References:
- Celli J, Zahrt TC. Mechanisms of Francisella tularensis Intracellular Pathogenesis. Cold Spring Harb Perspect Med. 2013; v.3(4):1-14.
- Dubey JP, Houk AE, Verma SK, Calero-Bernal R, Humphreys JG, Lindsay DS. Experimental transmission of Cystoisospora felis-like coccidium from bobcat (Lynx rufus) to the domestic cat (Felis catus). Vet Parasitol. 2015;211(1-2):35-39.
- Federal Select Agent Program, US Department of Health and Human Services, accessed May 31, 2022.
- Friend M. Tularemia. Circular 1297. U.S. Geological Survey; 2006
- Hirschmann JV. From Squirrels to Biological Weapons: The Early History of Tularemia. Am J Med Sci. 2018; 356(4): 319-328.
- Keim P, Johansson A, Wagner DM. Molecular epidemiology, evolution, and ecology of Francisella. Ann N Y Acad Sci. 2007;1105:30-66.
- Lamps LW, Havens JM, Anders S, Page DL, Scott MA. Histologic and molecular diagnosis of tularemia: a potential bioterrorism agent endemic to North America. Mod Pathol.2004;17:489-495.
- Maxie MG, Jubb KVF, Kennedy PC, Palmer N. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Elsevier Saunders; 2007.
- Petersen JM, Mead PS, Schriefer ME. Francisella tularensis: an arthropod-borne pathogen. Vet Res. 2009;40(2):7.
- Quinn PJ, Markey BK, Leonard FC, FitzPatrick ES, Fanning S, Hartigan PJ. Veterinary Microbiology and Microbial Disease. John Wiley & Sons; 2011.
- Timofeev V, Titareva G, Bahtejeva I, et al. The comparative virulence of Francisella tularensismediasiatica for vaccinated laboratory animals. Microorganisms. 2020;8(9):1403.
- World Health Organization. WHO Guidelines on Tularemia. WHO Press; 2007.
- Ziveri J, Barel M, Charbit A. Importance of Metabolic Adaptations in Francisella Front Cell Infect Microbiol. 2017; 7(96): 1-8.