Signalment:
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Histopathological examination of the systemic organs from this bird revealed necrotic changes and nonsuppurative inflammation in the heart, pancreas and brain that were consistent with HPAI infection lesion.(6) Immunohistochemical analysis using anti-H5 subtype influenza virus polyclonal antibody also revealed the distribution of viral antigens in myocardium, necrotic foci in pancreas and in astrocytes and neurons in the brain, suggesting systemic infection of influenza virus.Â
In domestic fowls, an experimental study found HPAI infection causes rapid viremia and produces vascular endothelial cell injury.(2,4) Necrotic and apoptotic changes of parenchymal cells of organs follow the endothelial damages. The most severely affected tissues are brain, heart, lungs and pancreas as well as primary and secondary lymphoid organs.(2,6) But the lesions vary from mild to severe, although the affected birds usually die shortly after systemic infection. The histopathological lesions in the present case were relatively severe.Â
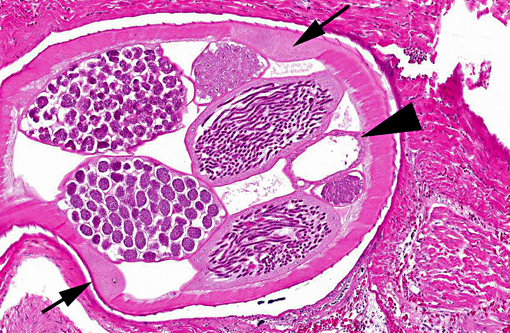
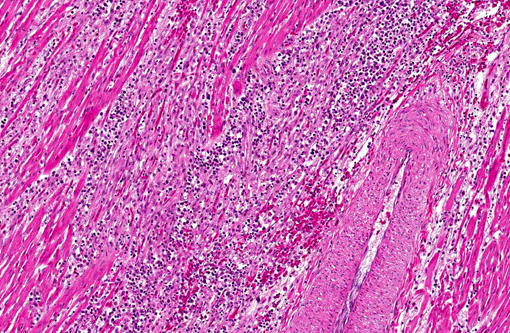
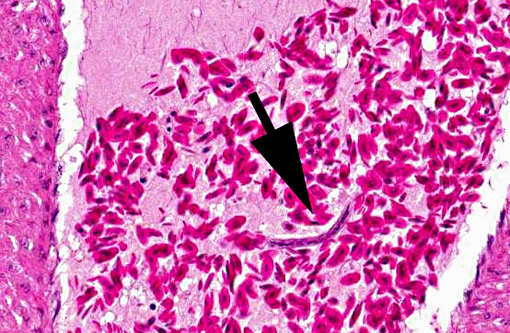
Adult heartworms containing microfilariae in their uterus were found in the heart of the present case. The morphology of the parasites presumably identified as Sarconema eurycerca, a filarial nematode of the superfamily Filariodea. Several studies of S. eurycerca infection in wild swans and geese were reported.(1,7) Although mortality cases in wild birds are reported, the pathological significance of this worm is still not well understood. Grossly, an enlargement of the heart with dark red and white streaks due to the migration of adult worms in myocardium is described in previous reports. The heart failure is induced by myocardial necrosis when numbers of parasites infected. Hemorrhage, lymphocytic and histiocytic inflammation was multifocally observed. Migration of microfilaria into myocardium, which is accompanied with inflammation, is sometimes observed, but was not observed in the present case. The infection of heartworms may partly contribute to develop severe myocardial lesions, but the cause of death in this swan might be concluded as systemic influenza virus infection.Â
JPC Diagnosis:
1. Heart: Myocarditis, heterophilic and granulomatous, chronic, multifocal, moderate, with myofiber necrosis, atrophy and loss, with adult filarid nematodes and microfilaria.
2. Heart: Vasculitis, heterophilic and necrotizing, multifocal, with thrombosis and myofiber degeneration and necrosis.Â
Conference Comment:
HPAI is in the family Orthomyxoviridae, genus Influenzavirus A. Influenza A viruses commonly infect horses, swine, and domestic poultry, as well as humans.(3) Wild birds, particularly ducks, shorebirds and gulls, are reservoir hosts for low-pathogenicity influenza A viruses; however, mink, seals, whales and dogs can also become infected. Influenza A viruses are currently classified into 16 hemagglutinin (H) and 9 neuraminidase (N) types. Gene reassortment results in numerous combinations of H and N subtypes; however, only a few combinations are important in naturally occurring infections in animals. Enzootic H7N7 and H3N8 viruses (previously called equine influenza viruses 1 and 2) cause respiratory disease in horses. Enzootic H1N1, H2N2, and H3N2 viruses affect swine. H3N8 and H3N2 viruses cause respiratory disease in dogs. Sporadic H10N4 viruses cause respiratory disease in mink. Sporadic H7N7 and H4N5 viruses cause disease in seals. In humans, H1N1, H2N2, H3N2, H5N1, H7N3, H7N7 and H9N2 viruses can all cause respiratory disease and virtually all of these types can be found in wild aquatic birds and domestic poultry. Of these, the H5 and H7 viruses can be associated with the high-pathogenicity phenotype. High pathogenicity is associated with changes in an amino acid sequence at the cleavage site of the hemagglutinin protein. These changes, which result in altered virulence, include the elimination of the glycosylation site, pH changes, and insertions or deletions in the amino acid sequence. While low pathogenicity subtypes are restricted to the intestinal and respiratory system in birds, highly pathogenic strains cause systemic disease characterized by viremia, endothelial injury and subsequent necrosis in multiple organs, as described by the contributor.(3)
Conference participants also discussed differential etiologic diagnoses for mononuclear myocarditis in birds including West Nile Virus, Newcastle Disease Virus, and protozoal myocarditis. As pointed out by the conference moderator, the presence of myocardial, pancreatic and/or hepatic necrosis in waterfowl should immediately raise the suspicion of HPAI infection.Â
References:
2. Kobayashi Y, Horimoto T, Kawaoka Y et al. Pathological studies of chickens experimentally infected with two highly pathogenic avian influenza viruses. Avian Pathol. 1996;25:285-304.
3. MacLachlan NJ, Dubovi EJ. Orthomyxoviridae. In: Fenners Veterianry Virology. 4th ed. New York, NY: Elsevier; 2011:353-370.
4. Park CH, Ozaki H, Takada A et al. Primary target cells of virulent strains of type A influenza virus in chicken embryos. Avian Pathol. 2001;30:269-272.Â
5. Sims LD, Domenech J, Benigno C, et al. Origin and evolution of highly pathogenic H5N1 avian influenza in Asia. Vet Rec. 2005;157:159-164.Â
6. Swayne DE, Halvorson DA. Influenza. In: Saif YM, Barnes HJ, Fadly AM, Glison JR, McDougald LR, Swayne DE, eds. Diseases of Poultry. 11th ed. Ames, IA: Iowa State University Press; 2003:147-149.
7. Woo GH, Jean YH, Bak EJ, Kang S, Roh IS, Lee KH, et al. Myocarditis by nematodes infection, presumably Sarconema eurycerca, in a wild whooper swan (Cygnus Cygnus) in Korea. J Vet Med Sci. 2010;72:1233-1235.