Wednesday Slide Conference, 2025-2026, Conference 11, Case 3
Signalment:
4.5-year-old, female, Holstein-cow, Bos taurusHistory:
Gross Pathology: In all submitted tissues, multifocal to coalescing, miliary to nodular, whitish, coarsely textured foci were noted on the cut surface.Laboratory Results:
N/AMicroscopic Description:
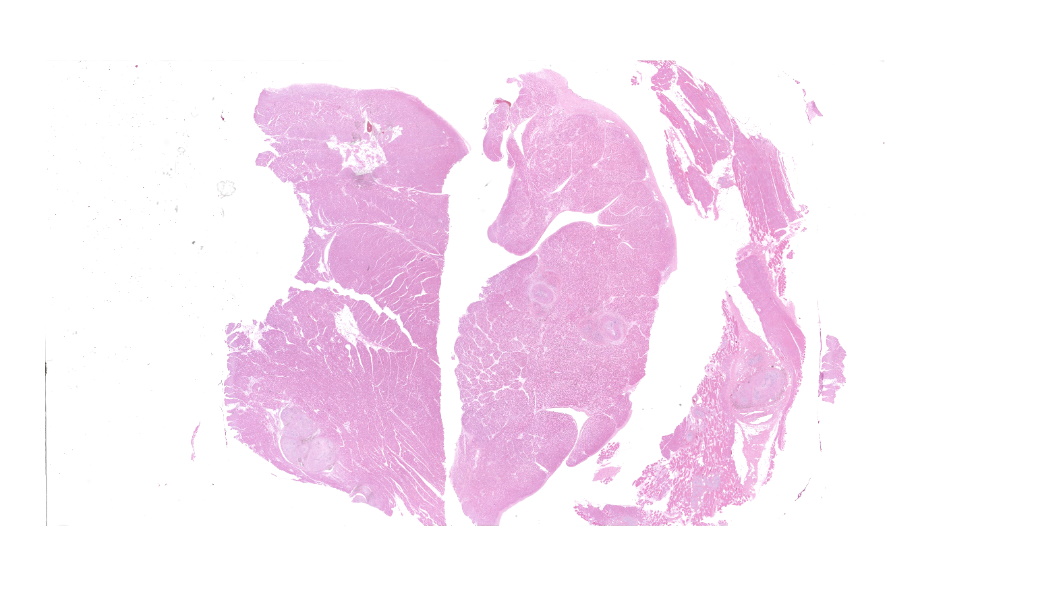
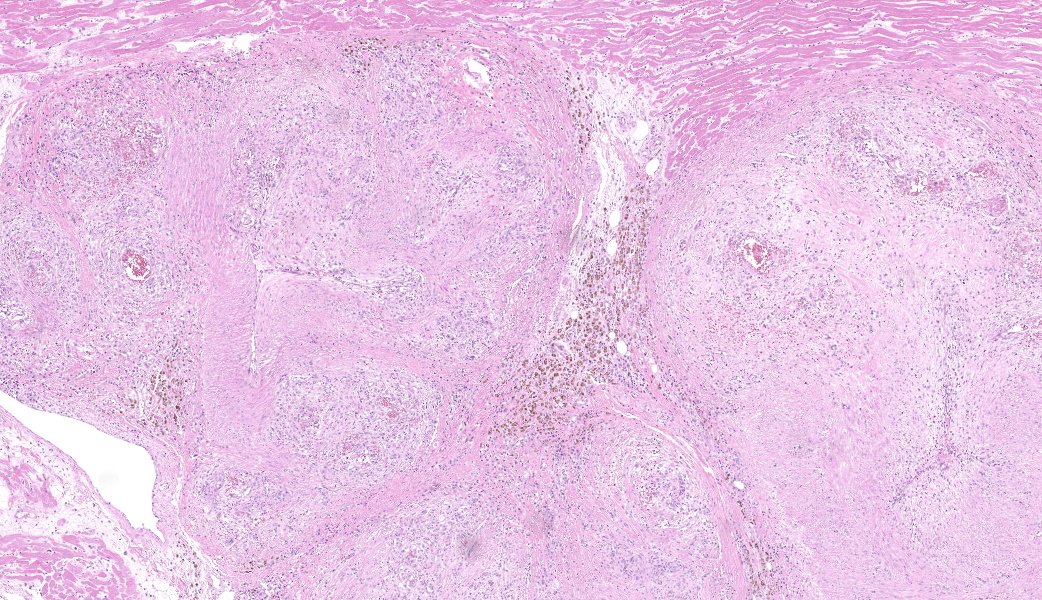
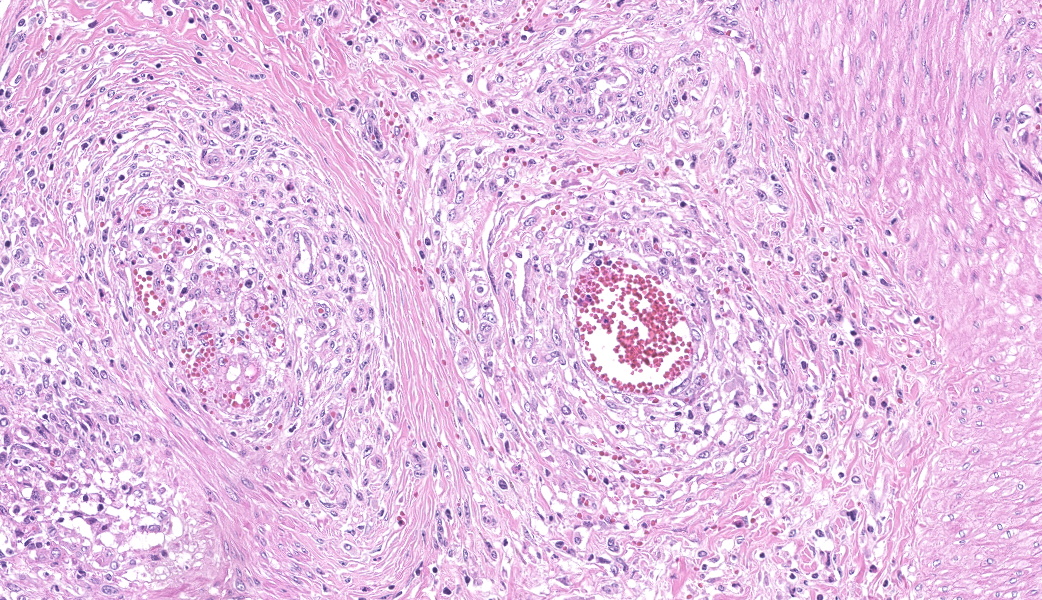
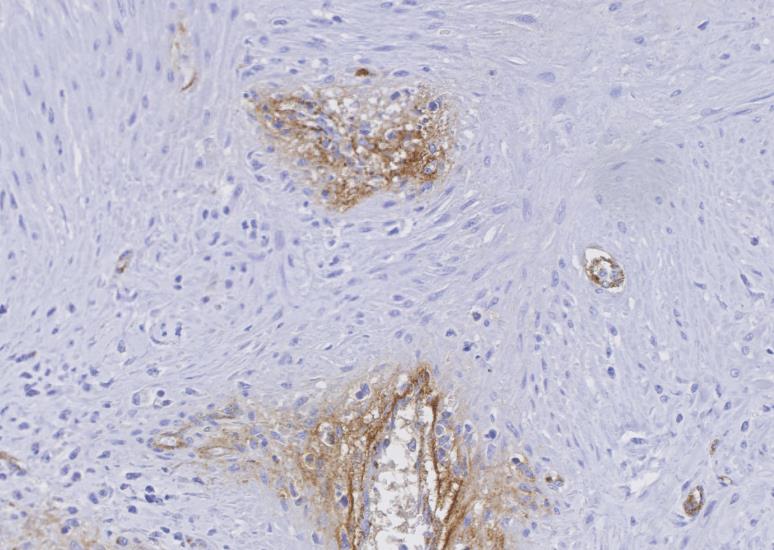
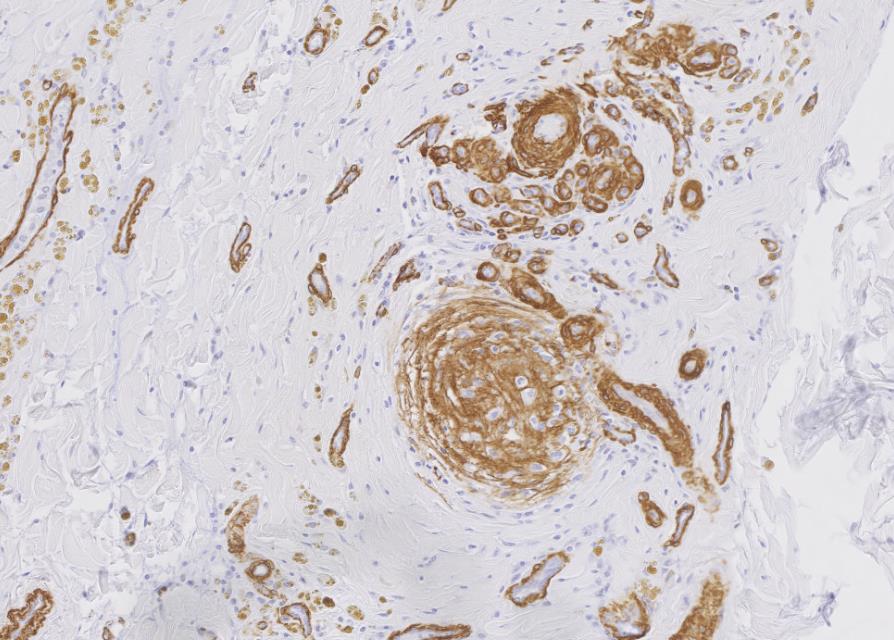
Myocardium (left ventricular wall) and striated muscle (brisket): Affecting approximately 10-20% of the myocardial and skeletal muscle tissue, there is a marked intraluminal, haphazardly arranged, often glomeruloid, highly cellular proliferation of plump spindeloid cells within medium to large-size arterioles with partial to complete occlusion of the vascular lumina accompanied by a pronounced thickening of the corresponding tunica media (medial hypertrophy). These spindeloid cells have abundant, slightly granular, eosinophilic, occasionally vacuolized cytoplasm with indistinct cell borders containing oval to cigar-like shaped nuclei with inconspicuous nucleoli and coarsely stippled chromatin. There is moderate anisokaryosis and anisocytosis with infrequent multinucleated cells being present. Mitotic figures are rare or absent (<1/HPF).There are frequent perivascular accumulations of variable numbers of hemosiderinloaden macrophages intermingled with a low number of inflammatory cell infiltrates consisting of lymphocytes and plasma cells as well as fewer neutrophils with rare small aggregates of extravasated erythrocytes. Few affected arterioles are infiltrated by a small to moderate number of eosinophils (not present on all slides). These inflammatory infiltrates also extend occasionally into the adjacent myocardial interstitium. The glomeruloid proliferations in the heart are surrounded by a moderate to marked deposition of collagen fibers (fibrosis) along with edema. Occasionally and affecting less than 5% of cardiomyocytes, there are intrasarcoplasmic protozoal cysts of approximately 100-200 µm in diameter containing numerous crescent-shaped bradyzoites. Perilesional myocytes of the brisket often display sarcoplasmic hypereosinophilia, swelling, loss of cross-striation and occasional fragmentation (hyaline degeneration and necrosis). Immunohistochemical labelling shows frequent cytoplasmic reactivity of the intralesional spindeloid cells for factor VIII/von Willebrand factor (vWF) and smooth muscle actin (SMA).
Contributor's Morphologic Diagnoses:
Myocardium of the left ventricular wall and striated muscle (brisket): Severe multifocal arteriolar intraluminal endothelial and pericyte proliferation with intraand perilesional hemosiderosis as well as hemorrhages accompanied by diffuse media hypertrophy (consistent with reactive angioendotheliomatosis) and mild eosinophilic arteriolitis Heart (myocardium): Moderate to marked, multifocal to coalescing interstitial lymphoplasmacytic myocarditis and myocardial fibrosis with occasional intrasarcoplasmic protozoal cysts (most likely Sarcocystis sp.) Striated muscle (brisket): Mild multifocal degeneration and necrosis of myocytesContributor's Comment:
In human medicine, the term angioendotheliomatosis includes either a malignant or a benign variant. The latter, also termed as reactive angioendotheliomatosis (RAE), is characterized by intravascular endothelial as well as pericyte proliferation and is mainly restricted to the skin.9,16 Associations between this condition and type 2, 3, or 4 hypersensitivity diseases were hypothesized, including autoimmune diseases, such as thrombotic thrombocytopenic purpura (TTP), underlying subacute systemic infections, or organ transplantation.8,11,14,16 The malignant angioendotheliomatosis (MAE) commonly refers to a rare type of angiotropic large cell lymphoma but may also apply to intravascular disseminated angiosarcoma.9,16In veterinary medicine, cases of MAE in the form of intravascular angiotropic lymphoma have been documented in dogs and to a lesser extent in cats.7,10 All previously published cases had concurrent involvement of the central nervous system and, to a lesser extent, other organs of the digestive, endocrine, respiratory, and urogenital systems of which the liver, lung, small intestine, and kidney are frequently represented. The veterinary cases mostly resembled human reports except for humans mainly being affected by a B-cell lineage neoplasm.10
Contrary to humans in which RAE is mainly benign, the feline counterpart feline systemic reactive angioendotheliomatosis (FSRA) is a rare fatal multisystemic disease of unclear origin with few documented cases.3,12,13,16 Affected animals often present with cardiorespiratory symptoms due to the heart being the main affected organ. Other organs in which lesions were frequently observed include the kidney, spleen, intestine, and lymph nodes, followed by others, but to a lower degree.3,11 In most of these cases, the cause remained unidentified. One cat was diagnosed with TTP, to which the lesions were presumably attributed.2
Previously, there has been one report in which such proliferative lesions were observed in a 2-year-old Corriente steer. The lesions resembled FRSA and were termed systemic reactive angioendotheliomatosis-like syndrome.2 The authors believed that the animal was persistently infected with the bovine viral diarrhea virus (BVDV) since they detected corresponding intralesional antigen by immunohistochemistry. The authors hypothesized that a TTP-like condition due to a BVDV infection was present.2 Formalin-fixed paraffin-embedded tissues (FFPE) of this animal plus other species suffering from vascular proliferative disorders, including cats with FSRA, were subjected to molecular analysis yielding the isolation of Bartonella sp.-DNA in all specimens. In addition, the authors demonstrated in vitro evidence for Bartonella vinsonii sp. berkhoffi being capable of inducing the transcription factor hypoxia-induced factor-1 (HIL-1) with subsequent production of the endothelial mitogen vascular endothelial growth factor (VEGF) in human cells (HeLa 229).1 The results led to the conclusion that there might be an association between Bartonella sp.-infections and the vascular proliferative disorders. However, these results need to be interpreted with caution as there is a likelihood of false-positive PCR results for Bartonella spp. in FFPE-specimens due to cross-contamination during necropsy or histology processing.15
The findings of the present case are consistent with the case report of Breshears et al., and the diagnosis of a systemic reactive angioendotheliomatosis-like syndrome was made. The glomeruloid proliferation of endothelial cells and pericytes staining positive for factor VIII/von Willebrand factor (vWF) and smooth muscle actin (SMA), respectively, is in concordance with the previous literature on RAE and FSRA.2,3,10,16,17 Without exception, these lesions were present in all submitted tissue samples including the kidney and flank muscle (not shown). No evidence for the presence of BVDV antigen was detected by immunohistochemical labeling using the C42 and 15C5 monoclonal antibodies against the viral protein Erns (envelope protein, RNase, secreted) of which one is specifically directed against BVDV while the other is detecting pestiviruses, respectively.4 Furthermore, no intralesional argyrophilic microbial structures could be found.
The occasionally seen intra-sarcoplasmic protozoal cysts are most likely bradyzoites of a Sarcocystis sp. In brief, this is a highly prevalent heteroxenous coccidian parasite, including around 90 species, which can infect a wide range of species, including humans. Its lifecycle consists of gametogeny (sexual stages) in the definitive host and schizogony (asexual stages) in the intermediate host. Coincidentally, the schizogony partially takes place within endothelial cells of arterioles and capillaries before its final stage in the musculature.6 On some slides, we noticed a mild eosinophilic intralesional inflammatory component which are most likely due to the protozoal infection. However, no additional investigation has been attempted in this case, as the significance of this infection is mostly clinically irrelevant.
Contributing Institution:
University of Zurich, Winterhurerstrasse 268https://www.vetpathology.uzh.ch
JPC Diagnoses:
- Heart and skeletal muscle, arterioles: Atypical endothelial and pericyte proliferation (angioendotheliomatosis), chronic, diffuse, severe, with medial hypertrophy and mural and adventitial fibrosis.
- Heart, myocytes: Sarcocysts, multiple. Another unique condition in its first WSC iteration in this species, this case also proved to be diagnostically challenging for participants. The contributor provided an excellent write-up summarizing the current literature on this condition across species. Similar cases of systemic reactive angioendotheliomatosis and SRE-like syndrome have been seen before in the WSC (Conference 21, Case 3, 2014, Conference 13, Case 4, 2019, and Conference 9, Case 1, 2020), all of which have had great write-ups and similar immunohistochemical staining patterns with vWF and smooth muscle actin (SMA) to those that were performed in this case, but were all in cats.
- The contributor’s comment covered all pertinent aspects of conference discussion, from the comparison between human and veterinary forms of this condition, to the current hypotheses on this syndrome’s pathogenesis, to the possible associations of this condition with TTP and certain systemic infections (i.e. Bartonella spp. in cats and bovine pestivirus in cattle). Although this condition is rare, a recent 2024 paper found that systemic reactive angioendotheliomatosis in cats can mimic hypertrophic cardiomyopathy both clinically and on echocardiogram and should be considered as a differential for cats that have symmetrical left ventricular wall thickening.5
JPC Comment:
Another unique condition in its first WSC iteration in this species, this case also proved to be diagnostically challenging for participants. The contributor provided an excellent write-up summarizing the current literature on this condition across species. Similar cases of systemic reactive angioendotheliomatosis and SRE-like syndrome have been seen before in the WSC (Conference 21, Case 3, 2014, Conference 13, Case 4, 2019, and Conference 9, Case 1, 2020), all of which have had great write-ups and similar immunohistochemical staining patterns with vWF and smooth muscle actin (SMA) to those that were performed in this case, but were all in cats.The contributor’s comment covered all pertinent aspects of conference discussion, from the comparison between human and veterinary forms of this condition, to the current hypotheses on this syndrome’s pathogenesis, to the possible associations of this condition with TTP and certain systemic infections (i.e. Bartonella spp. in cats and bovine pestivirus in cattle). Although this condition is rare, a recent 2024 paper found that systemic reactive angioendotheliomatosis in cats can mimic hypertrophic cardiomyopathy both clinically and on echocardiogram and should be considered as a differential for cats that have symmetrical left ventricular wall thickening.5
References:
- Beerlage C, Varanat M, Linder K, Maggi RG, Cooley J, Kempf VA, Breitschwerdt EB. Bartonella vinsonii subsp. berkhoffii and Bartonella henselae as potential causes of proliferative vascular diseases in animals. Med Microbiol lmmunol. 2012;201(3):319-326.
- Breshears MA, Johnson BJ. Systemic reactive angioendotheliomatosis-like syndrome in a steer presumed to be persistently infected with bovine viral diarrhea virus. Vet Pathol. 2008;45(5):645-649.
- Fuji RN, Patton KM, Steinbach TJ, Schulman FY, Bradley GA, Brown TT, Wilson EA, Summers BA. Feline systemic reactive angioendotheliomatosis: eight cases and literature review. Vet Pathol. 2005;42(5): 608-617.
- Herrold E, Schober K, Miller J, Jennings R. Systemic reactive angioendotheliomatosis mimicking hypertrophic cardiomyopathy in a domestic shorthair cat. J Vet Cardiol. 2024;56:65-71.
- Hilbe, Camenisch, Braun, Peterhans, Stalder, P, Zlinszky, & Ehrensperger. Mucosal lesions in a sheep infected with the Border Disease Virus (BDV). Schweizer Archiv für Tierheilkunde. 2009;151(8), 391-396.
- Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. St. Louis, MO: Elsevier; 2016:235-6.
- Lapointe JM, Higgins RJ, Kortz GD, Bailey CS, Moore PF. lntravascular malignant T-cell lymphoma (malignant angioendotheliomatosis) in a cat. Vet Pathol. 1997;34(3):247-250.
- Lazova R, Slater C, Scott G. Reactive angioendotheliomatosis. Case report and review of the literature. Am J Dermatopathol. 1996;18(1):63-69.
- Lin BT, Weiss LM, Battifora H. lntravascularly disseminated angiosarcoma: true neoplastic angioendotheliomatosis? Report of two cases. Am J Surg Pathol. 1997;21(10):1138-1143.
- McDonough SP, Van Winkle TJ, Valentine BA, vanGessel YA, Summers BA. Clinicopathological and immunophenotypical features of canine intravascular lymphoma (malignant angioendotheliomatosis). J Comp Pathol. 2002;126(4):277-288.
- McMenamin ME, Fletcher CD. Reactive angioendotheliomatosis: a study of 15 cases demonstrating a wide clinicopathologic spectrum. Am J Surg Pathol. 2002;26(6):685-697.
- Rothwell TL, Xu FN, Wills EJ, Middleton DJ, Bow JL, Smith JS, Davies JS. Unusual multisystemic vascular lesions in a cat. Vet Pathol. 1985;22(5):510-512.
- Straumann Kunz U, Ossent P, Lott-Stolz G. Generalized intravascular proliferation in two cats: endotheliosis or intravascular pseudoangiosarcoma? J Comp Pathol. 1993;109(1):99-102.
- Umlas, J. Glomeruloid structures in thrombohemolytic thrombocytopenic purpura, glomerulonephritis, and disseminated intravascular coagulation. Human Pathology. 1972;3(3):437-441.
- Varanat M, Maggi RG, Linder KE, Horton S, Breitschwerdt EB. Cross-contamination in the molecular detection of Bartonella from paraffin-embedded tissues. Vet Pathol. 2009;46(5):940-944.
- Wick MR, Rocamora A. Reactive and malignant "angioendotheliomatosis": a discriminant clinicopathological study. J Cutan Pathol. 1988;15(5):260-271.
- Yamamoto S, Shimoyana Y, Haruyama T. A case of feline systemic reactive angioendotheliomatosis. J Fel Med Surg.