Signalment:
Gross Description:
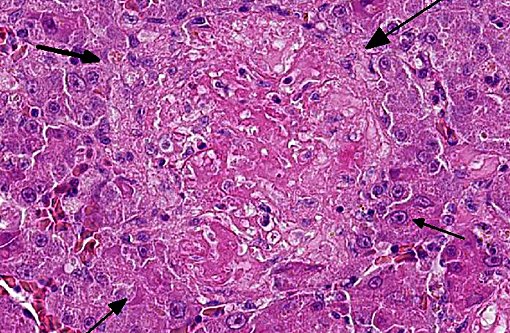
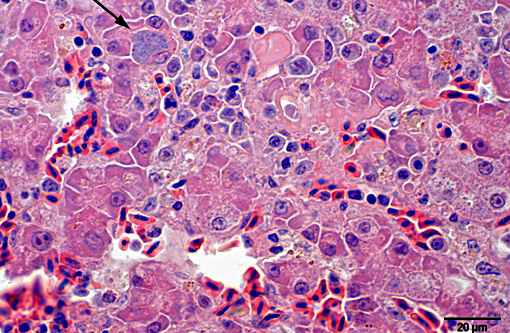
Histopathologic Description:
Giemsa staining revealed multifocal dark purple cytoplasmic inclusions in both hepatocytes and macrophages.
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Infection with C. psittaci occurs primarily via inhalation, ingestion or conjunctival exposure to contaminated feather dust or faeces. The bacteria spread to lung, air sacs and pericardial sac within few hours. EBs attach to microvilli of the host cell membrane and are internalized via invagination.(4) Bacteria-containing endocytic vesicles are called inclusions. They proceed to the nuclear area where EBs differentiate into RBs. A role of snapin (a part of the SNARE complex) connecting chlamydial inclusions with the microtubule network by interacting with both IncB and dynein has been proposed.(1) RBs replicate via binary fission and reorganize, through IBs, into new EBs. New EBs are released from host cell via cell lysis or exocytosis. In high pathogenic serotypes the inclusion membrane degrades mainly during the active multiplication, releasing the bacteria into the cytoplasm, leading to cell lysis.(7) The rapid multiplication leads to bacteraemia within 48 hours. Infectious EBs are spread via cloaca and/or nasal turbinates into the environment.
Typical clinical findings include diarrhoea and excretion of green to yellow-green urate. Severely affected birds may become anorectic and produce sparse, dark green droppings, followed by emaciation, dehydration and death.
Typical gross findings are hepatomegaly and splenomegaly with or without necrotic foci, often accompanied by fibrinous airsacculitis, pericarpditis and peritonitis and serous to purulent conjunctival exudate.
The genus Chlamydophila contains six species: C. abortus (ruminants), C. caviae (guinea pigs), C. felis (felidae), C. pecorum (ruminants, swine, koalas), C. pneumonia (humans, marsupialia and amphibia) and C. psittaci (birds). Chlamydophila spp. are known to infect at least 469 domestic, free-living or pet bird species in 30 orders.(3) Of the nine known outer membrane protein A (ompA) subtypes of C. psittaci, seven are known to naturally infect birds (A F and E/B) and two to infect mammals (M56 muskrat and snowshoe hare, WC cattle). Each avian serotype seems to be associated with a different group or order of birds.(2) Furthermore, strains of C. psittaci fall into two general categories: highly or less virulent strains. Highly virulent strains or toxigenic strains (e.g. serotype D) are isolated most often from turkeys and occasionally from clinically inapparent wild birds. In natural and experimental hosts, they are responsible for a rapid and fatal disease progression characterized by extensive vascular congestion and inflammation of vital organs. Veterinarians and poultry workers are especially at risk of becoming infected with serovar D strains. Strains of low virulence (e.g. serotype B and E) cause slowly-progressing epidemics with a mortality rate of less than 5% and neither develop severe vascular damage nor severe clinical signs.(6) They are routinely isolated from pigeons and ducks. Chlamydiosis may be accompanied by concurrent infection with Salmonella spp, especially in pigeons, ducks and some psittacine birds. Avian strains can infect humans and may lead to severe pneumonia. However, cases of human-to-human transmission rarely occur. Immunity to chlamydia is generally poor and short-lived. Older birds are often more susceptible to clinical signs than younger ones.
JPC Diagnosis:
Conference Comment:
All genotypes / subtypes of C. psittaci can be transmitted to people and cause psittacosis (i.e. parrot fever), a disease originally named when infection was thought to be only transmitted from psittacine birds. Most cases of human psittacosis are related to contact with psittaciformes; however, it is also an occupational hazard for those working in the poultry industry and others in close contact with different avian species.(8) Infection in humans can result in a wide range of disease from asymptomatic infections to mild influenza-like symptoms, to severe disease with pulmonary and extrapulmonary involvement including gastrointestinal, hepatic, cardiac and neurologic signs.
C. psittaci infection in poultry production operations is most commonly associated with turkey or duck farms, but has also been documented in chickens, more commonly broilers. In chickens, both the ompA genotypes B and the more pathogenic D can cause clinical infection consisting of respiratory symptoms. However, as described above for other avian species, respiratory symptoms are generally more severe in genotype D infected chickens and it may also result in anorexia and mortality. Additionally, genotype D shows significantly higher bacterial replication in infected tissues and may demonstrate systemic dissemination. It is found to be excreted in the cloaca and from the pharynx in infected chickens as seen in other avian species.(9)
Atherosclerosis is frequently seen in captive psittacine birds and is considered the most common vascular disease of birds. Interestingly, it has been associated with the presence of C. psittaci antigen in blood vessels indicating infection may be a risk factor for development of atherosclerosis.(5) However, the exact role of C. psittaci infection in the pathogenesis of atherosclerosis, regarding severity and strain, is unclear.
References:
1. B+â-¦cker S, Heurich A, Franke C, et al. Chlamydia psittaci inclusion membrane protein IncB associates with host protein Snapin. Int J Med Microbiol. 2014;304(5-6):542-53.
2. Geens T, Desplanques A, Van Loock M, et al. Sequencing of the Chlamydophila psittaci ompA gene reveals a new genotype, E/B, and the need for a rapid discriminatory genotyping method. J Clin Microbiol. 2005;43(5):2456-61.
3. Kaleta EF, Taday EMA. Avian host range of Chlamydophila spp. based on isolation, antigen detection and serology. Avian Pathol. 2003;32(5):435-462.
4. Hodinka RL, Wyrick PB. Ultrastructural study of mode of entry of Chlamydia psittaci into L-929 cells. Infect Immun. 1986;54(3):855-63.
5. Pilny AA, Quesenberry KE, Bartick-Sedrish TE, et al. Evaluation of Chlamydophila psittaci infection and other risk factors for atherosclerosis in pet psittacine birds. J Am Vet Med Assoc. 2012;240(12): 1474-80.
6. Tappe JP, Andersen AA, Cheville NF. Respiratory and Pericardial Lesions in Turkeys Infected with Avian or Mammalian Strains of Chlamydia psittaci. Vet Pathol. 1989;26(5):386-395.
7. Vanrompay D, Charlier G, Ducatelle R, et al. Ultrastructural changes in avian Chlamydia psittaci serovar A-, B-, and D-infected Buffalo Green Monkey cells. Infect Immun. 1996;64(4): 1265-71.
8. Vanrompay D, Harkinezhad T, van de Walle M, et al. Chlamydophila psittaci transmission from pet birds to humans. Emerg Infect Dis. 2007;13(7):1108-1110.
9. Yin L, Lagae S, Kalmar I, et al. Pathogenicity of low and highly virulent Chlamydia psittaci isolates for specific-pathogen-free chickens. Avian Diseases. 2013;57(2):242-247.