Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 2
28 August 2019
CASE IV: 14/1790 (JPC 4117447).
Signalment: 13-year-old spayed female Labrador retriever dog, Canis familiaris, canine
History: This dog presented for a mass on the vaginal floor. Complete blood count and chemistry were unremarkable.
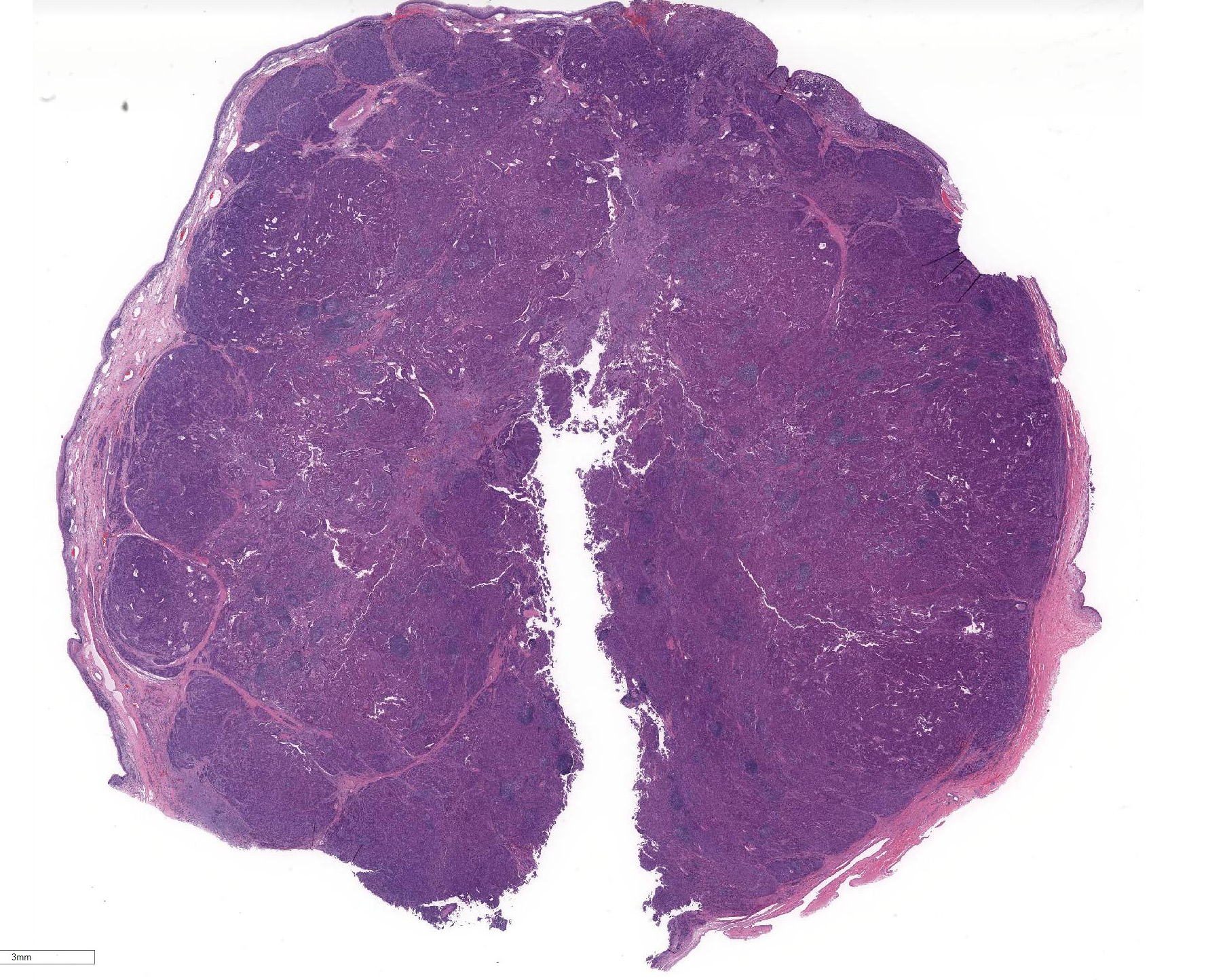
Gross Pathology: 3cm diameter, asymmetrical, lobulated and highly vascular mass protruding from the vaginal floor.
Laboratory results: None
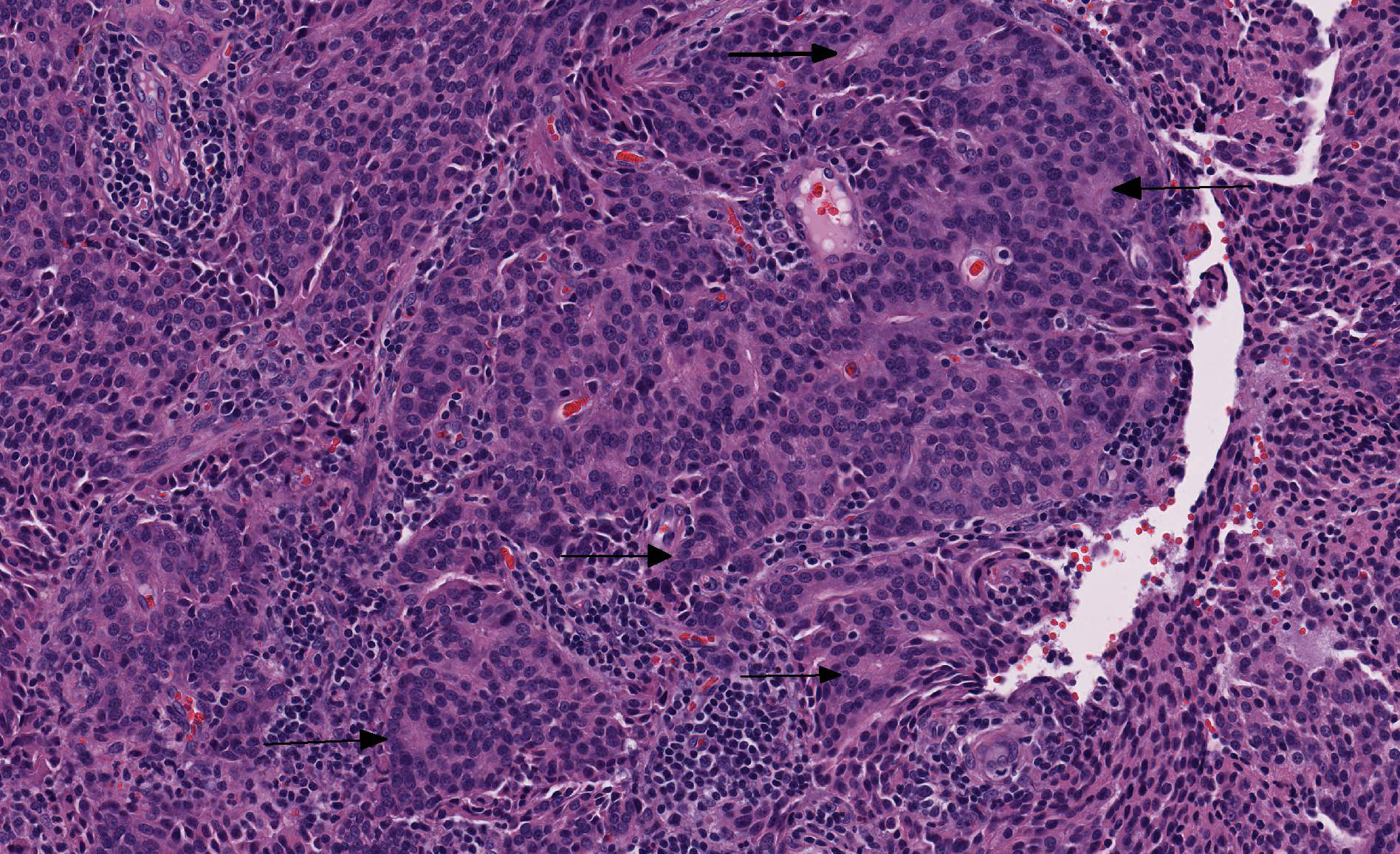
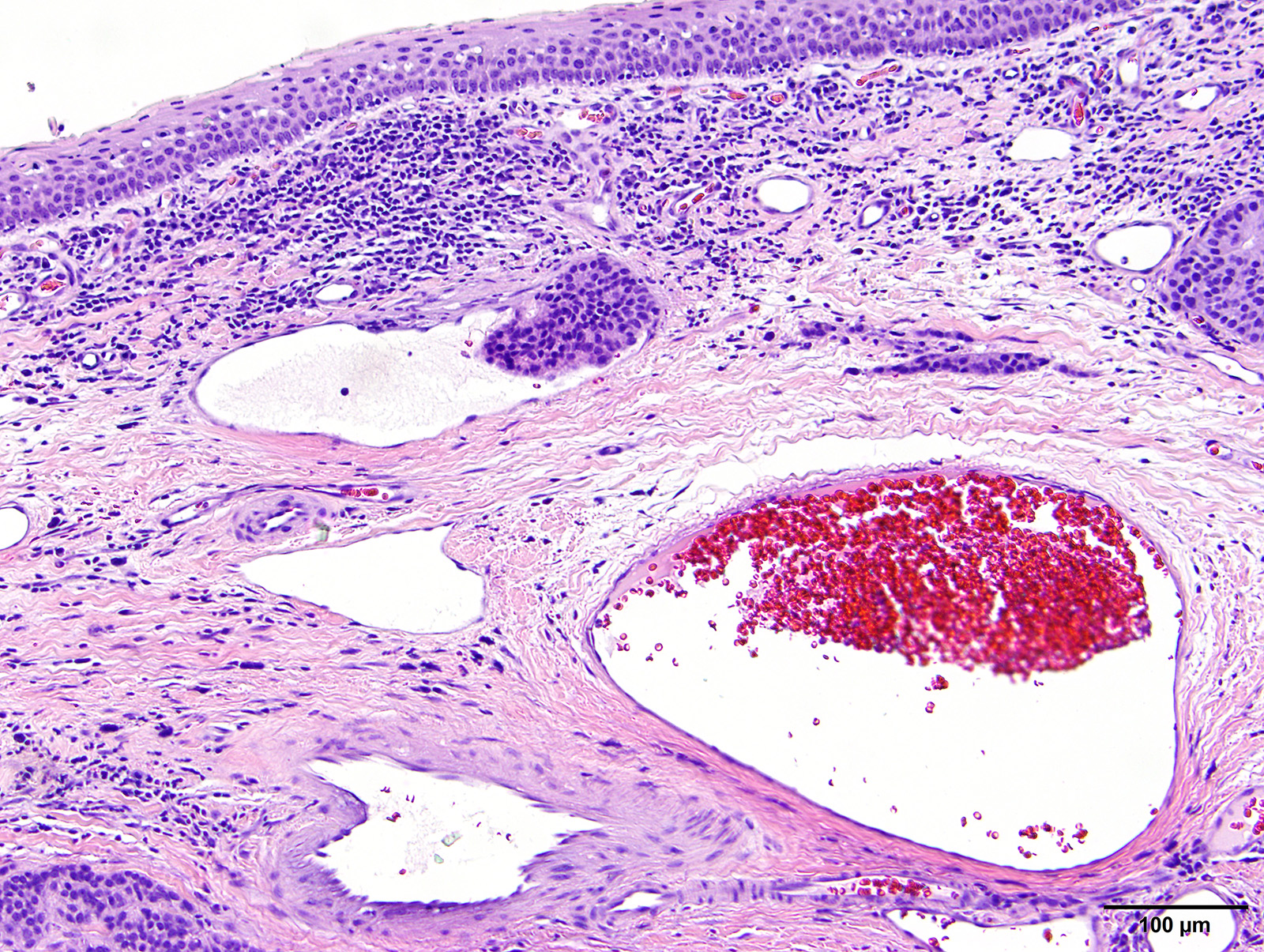
Microscopic Description: Vaginal floor mass: This is an expansile, fairly well-demarcated, unencapsulated, multilobulated and peripherally invasive neoplasm composed of cuboidal to polygonal cells arranged in cords, acini, occasional rosettes (Figure 1) and solid areas with fine fibrovascular stroma. Neoplastic cells have indistinct cell borders, a moderate amount of eosinophilic cytoplasm, and round to oval finely stippled nuclei with prominent nucleoli. There is mild anisocytosis and anisokaryosis and one mitotic figure in 10 high power (400x) fields. Rafts of neoplastic cells are within multiple endothelial lined vessels (vascular invasion) (Figure 2) [varies by slide]. Scattered throughout the mass are perivascular clusters of lymphocytes and foci of hemorrhage with hemosiderin-laden macrophages.
Contributor Morphologic Diagnoses: Clitoral gland adenocarcinoma
Contributor Comment: Vaginal and vulvar tumors are uncommon neoplasms which typically occur in older, sexually intact females.7 The majority of these neoplasms are benign and of smooth muscle or fibrocyte origin.7 Few, individual case reports of epithelial neoplasms resembling apocrine gland anal sac adenocarcinomas (AGASACA), but arising within the clitoral fossa, have been documented in the literature since 2010.3,4,5,8 A case series published in 2018 has added 6 more cases to the literature.8
Clitoral gland carcinomas are thought to arise from individual apocrine glands within the fibro-fatty tissues of the canine clitoris. They typically present as ulcerated, multilobular masses protruding from the vulva. Histologically, these tumors closely resemble their anal sac counterpart, and it is possible that tumors at this location have been previously reported as AGASACAs. Tubular, rosette, and solid forms are described in the paper by Verin et al.,8 although most tumors are mixed-type. Only carcinomas have been reported; a majority of cases have vascular invasion at the time of diagnosis.8 Tumors that have been stained with neuroendocrine markers are typically uniformly positive for neuron-specific enolase, with mixed positivity for chromogranin A and synaptophysin and negative for S-100.8
Interestingly, in two earlier reports of clitoral gland adenocarcinoma in the dog, hypercalcemia of malignancy (HM) was a feature.3,4 However, in the six cases reported recently, only 2 of 6 cases exhibited HM; one case confirmed an elevated parathormone-related hormone (PTHrp) as the likely cause.8
In this case, the animal received two doses of chemotherapy before treatment was stopped due to undesirable side effects. Although the neoplasm has recurred at the original site, there was no evidence of metastatic disease or hypercalcemia of malignancy more than 2 years after initial diagnosis and treatment.
Contributing Institution:
University of Tennessee, College of Veterinary Medicine, Department of Biomedical and Diagnostic Sciences http://www.vet.utk.edu/departments/path/index.php
JPC Diagnosis: Mass from vaginal floor: Clitoral gland adenocarcinoma.
JPC
Comment: The
contributor has done an outstanding job summarizing the histologic and
clinicopathologic features of this uncommon neoplasm of the vulva of the dog. A
2016 paper by Rout et al.5 describes the salient features of this
tumor on cytologic evaluation: loosely cohesive and acini of epithelial cells
with numerous "naked" nuclei in the surrounding milieu, suggestive of
neuroendocrine carcinoma. Cell clusters may include capillaries or pink amorphous
material.5 In addition, clitoral gland adenocarcinomas, like the
closely related tumors of the apocrine glands of the anal sac often metastasize
to regional nodes; close examination of regional nodes (including the inguinal
as well as medial and internal iliac nodes) for gross evidence of metastasis is
recommended.8
Neoplasms of the clitoral gland are well known in the rat and these glands, as
well as the preputial glands of males, are usually sampled in carcinogenicity
and xenobiotic studies in the mouse and rat.6 These modified
sebaceous glands are located adjacent to the vagina, and their ducts empty into
the clitoral fossa. Their growth and activity are regulated by a number of
hormones, including sex hormones, as well as ACTH, prolactin, and growth
hormones. In general, a range of lesions is seen in this gland similar to
those seen in the mammary gland. 6
In rodents, inflammatory changes of the clitoral gland may range from simple
lymphocytic infiltrates to actual inflammation resulting from bacterial or
fungal agents, or the presence of foreign bodies in adjacent tissue.
Obstructed clitoral gland ducts may result in granulomatous inflammation.
Hyperplasia, or less commonly atrophy, is seen in older animals. Clitoral
gland neoplasms are often not large enough to be observed grossly and necropsy,
so this tissue (as well as preputial gland, mammary gland, and the rat Zymbalâs
gland) should be routinely harvested at necropsy.6
A range of neoplasms and subtypes have been observed in rodents. Adenomas of
the clitoral glands, and are characterized as expansile growths with minimal
atypia and few mitoses. 6 Clitoral gland carcinomas are generally
larger with invasive growth, ulceration of the overlying skin, nuclear atypia
and a high mitotic rate. Several subtypes (solid, cystic, papillary, papillary
cystic, and mixed cell types) are described, but the current INHAND document
does not recommend subtyping for regulatory activities. Squamous papillomas and
malignant basal cell variants of clitoral gland adenocarcinomas are also
described.6
In humans, the clitoris is covered by squamous mucosal epithelium without a glandular component.2 The overwhelming majority of neoplasms arising in and around this organ in human are squamous cell carcinomas.1 As the clitoris and surrounding tissues possess lymphatic drainage, malignancies from other areas of the body will rarely metastasize to this area.1
The moderator
stated she chose this particular case as it not only is a new addition to the
WSC database, but also has been recently published in the veterinary literature
and would be very helpful for residents preparing for certifying examinations.
She reviewed the various type of rosettes seen in various tumors including
Homer-Wright (central region contains neuropil), Flexner-Wintersteiner (central
region largely empty except cytoplasmic extensions from tumor cells), ependymal
rosettes (central regions are empty), and pseudorosettes (surround a blood
vessel). She also reviewed the various type of neoplasms whose cytologic
appearance includes large numbers of "naked nuclei"' to include a variety of
neuroendocrine neoplasms, apocrine carcinomas of the anal sac, and clitoral
adenocarcinoma.
References:
1. DuPont NC, Mabuchi S, Ries S, Berman ML. Sclerosing ductal carcinoma of the clitoris with microcystic adnexal carcinoma-like features. J Cutan Pathol 2009: 359-361.
2. Kurman RJ, Ronnett BM, Sherman ME, Wilkinson EJ. Anatomy of the lower female reproductive tract. In: AFIP Atlas of Tumor Pathology, Series 4: Tumors of the Cervix, Vagina, and Vulva. ARP Press, Silver Spring, MD, p. 20.
3. Mitchell KE, Burgess DM, Carrigan, MJ. Clitoral adenocarcinoma and hypercalcemia in a dog. Aust Vet Pract 2012; 42:279-282.
4. Neihaus SA, Winter JE, Goring RL, Kennedy FA, Kiupel M. Primary clitoral adenocarcinoma with secondary hyperpcalcemia of malignancy in a dog. J. Am Anim Hosp Assoc 2010; 46(3):193-196.
5. Rout ED, Hoon-Hanks LL, Gustafson TL, Ehrhart EJ, MacNeill AL. What is your diagnosis: Clitoral mass in a dog. Vet Clin Pathol 2016; 197-198.
6. Rudmann D, Cardiff R, Chouinard L, Goodman D, Kuttler K, Marxfeld, Molinolo A, Treumann S, Yoshizawa K. Proliferative and nonproliferative lesions of the rat and mouse mammary, Zymbalsâs, preputial, and clitoral glands. Toxicol Pathol 2012; 49: 7S-39S.
7. Thatcher C, Bradley RK. Vulvar and vaginal tumors in the dog: a retrospective study. J Am Vet Med Assoc. 1983;183(6):690â692.
8. Verin R, Cian F, Stewart J, et al. Canine clitoral carcinoma: A clinical, cytologic, Histopathologic, Immunohistochemical, and Ultrastructural Study. Vet Pathol. 2018; epub ahead of print. DOI: 10.1177/0300985818759772