Wednesday Slide Conference, Conference 11, Case 1
Signalment:
A 4 year old intact Male Beagle dog (Canis familiaris).
History:
Presented for a 9 day history of hyporexia and regurgitation. Previous history of being treated for hepatozoonosis in April 2023 with relapse in May 2023. Radiographs revealed bilateral femoral periosteal bone reaction. Abdominal ultrasound found free fluid (which was frank blood determined via abdominocentesis) and multiple cavitated lesions in spleen and liver. An abdominal exploratory laparotomy was performed which found a ruptured splenic mass as well as two additional masses within the liver. Humane euthanasia was elected given uncertain prognosis and the remains were submitted for necropsy.
Gross Pathology:
Arising from the base of the heart immediately adjacent to the aorta are two ovoid, green to dark brown, smooth and firm nodules measuring 2 cm x 1 cm x 1 cm.
Within the abdominal cavity is approximately 32 mL of red, opaque, watery fluid. All lymph nodes are mildly to moderately enlarged, reaching up to 2 cm diameter with the abdominal lymph nodes most severely affected.
Laboratory Results:
Hepatozoon spp. RealPCR: Negative
Microscopic Description:
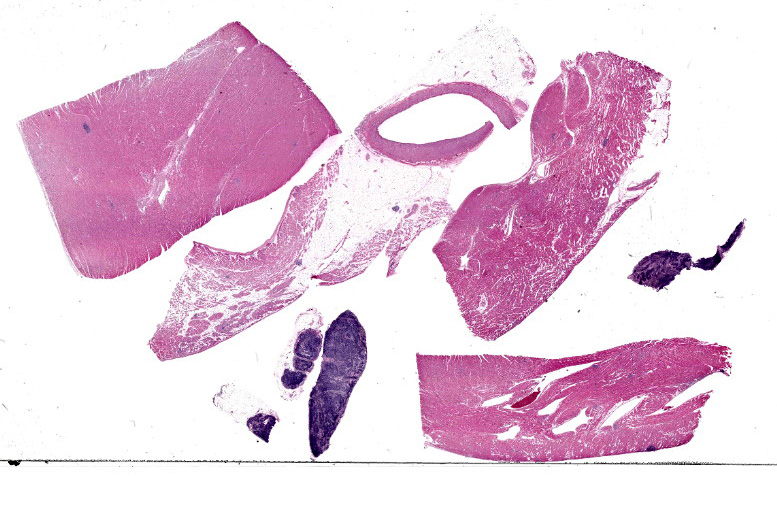
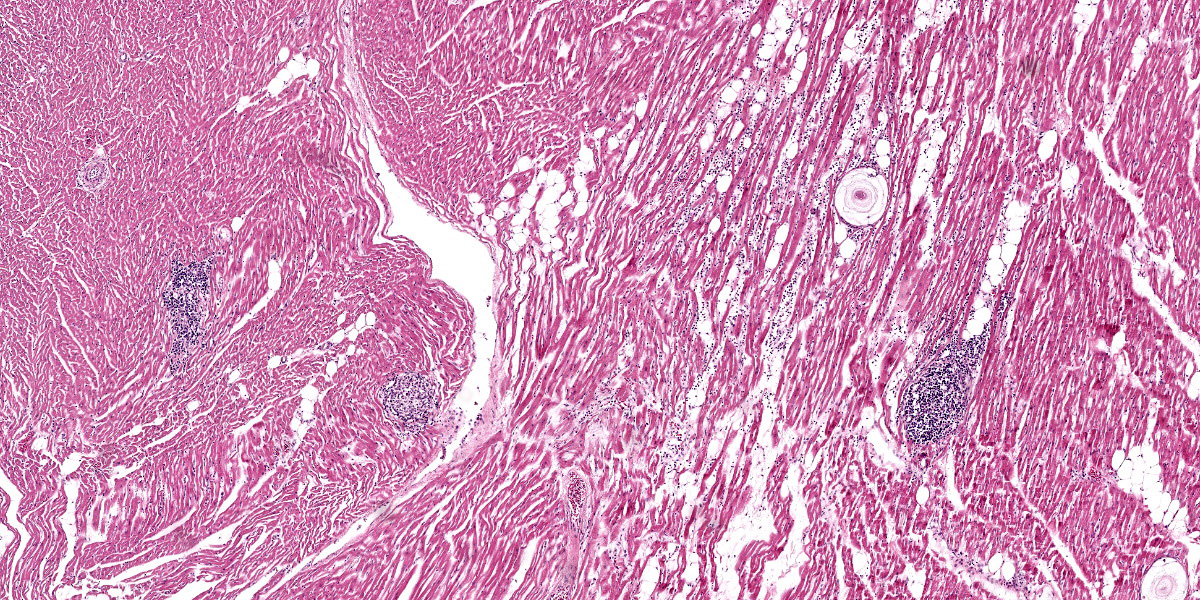
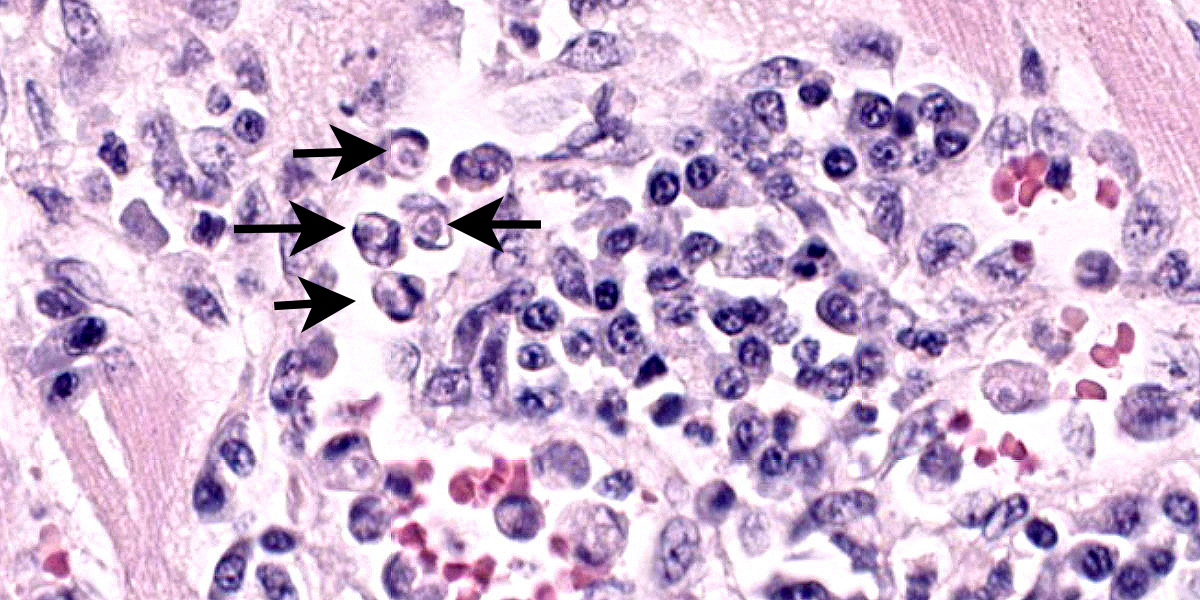
Heart: Examined are four sections. Multifocally infiltrating between and separating cardiomyocyte fibers are discrete, occasionally encapsulated, aggregates of predominantly lymphocytes and plasma cells with scattered macrophages and fibroblasts. Within several of these aggregates are reactive histiocytes containing spherical eosinophilic and intracytoplasmic round protozoan merozoites approximately 4-6μm in diameter and often displacing the host cell nuclei. Randomly distributed throughout the right, left, and interventricular septal walls are variably- sized multilamellar mucopolysaccharide rich cysts (Onion skin cysts, meronts) up to 200 μm in diameter. Occasionally within these maturing meronts are variable stages of merozoite development containing greater than 50, 2-3μm in diameter merozoites. These meronts frequently enmesh neighboring fibrocytes and are often surrounded by rings of leukocytes. Lymphocytes and plasma cells infiltrate between the adjacent
Contributor’s Morphologic Diagnosis:
Whole Body (Spleen, Lymph nodes, Heart, Blood vessels, Skeletal muscle): Chronic, multifocal lymphoplasmacytic and histiocytic inflammation with intralesional encysted protozoa (consistent with Hepatozoon sp.)
Contributor’s Comment:
The patient's reported progressive decline was considered multifactorial, likely owing to a combination of systemic hepatozoonosis and ruptured splenic hemangiosarcoma (not captured in the provided slide). The patient's hemoabdomen noted during the abdominal exploration surgery was subsequent to the ruptured splenic hemangiosarcoma as well as several ruptured hematomas along the hepatic parenchyma. These hepatic hematomas are presumed sequela to robust, hepatozoonosis-mediated hepatitis and hepatic necrosis with pronounced hepatic parenchymal loss.
Hepatozoon is a protozoan classified among the apicomplexan phylum, primarily affecting the canine species (intermediate host) in the southeastern and central United States with several species variants noted in Latin America, Africa, Asia, and Europe.1-9 Two primary species reported to infect domestic canids include Hepatozoon americanum and Hepatozoon canis while in felids Hepatozoon felis, Hepatozoon silvestris, and Hepatozoon canis are the primary species.1 Hepatozoonosis has additionally been documented in rodent, racoon, kiwis, opossums, and wild canids.1 Transmission of Hepatozoon spp. is via ingestion of arthropod vectors (direct host) with several tick species (e.g. Rhipicephalus, Amblyomma, and Ixodes spp. ) being well-documented.1-9 These ticks harbor the oocyst stage and upon ingestion release sporozoites into the host blood stream with subsequent deposition and infiltration in the spleen, bone marrow, lymph nodes, and major viscera where they encyst and form meronts.1-9 Merozoites begin to safely develop in these seemingly innocuous meronts and are released in mass upon complete maturation, thus inciting an aggressive (typically pyogranulomatous) host response.3 The true genius of this organism shines through, as these young merozoites safely hijack the recruited macrophages and neutrophils, escape the granuloma, and begin to undergo their final maturation into gamonts. Fully developed gamonts once again exit the leukocyte and re-enter host blood circulation to be once again consumed by another tick during a hematogenous meal and perpetuate its life cycle.1-9
The typical gross presentation is primarily restricted to skeletal and cardiac muscle manifesting as a nonspecific myositis and muscle wasting (as evidenced in this case) but can additionally involve major organs such as bone marrow, liver, lungs, and the kidneys. A striking but less frequently observed gross finding is periosteal proliferation of the long bones, which was not observed in this case.2,5
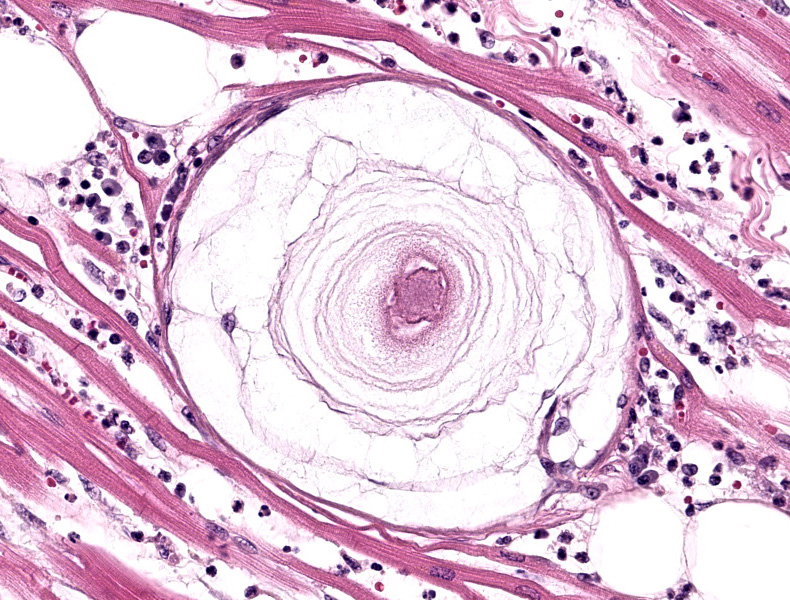
On microscopic examination, the diagnostic lesion is multiple encysted approximately 250μm in diameter meronts2 containing merozoites at varying stages of development enmeshed in a multilayered mucopolysaccharide and fibroblast-rich capsule (onion-skin cyst).3 In systemic infection, leukocytes (macrophage, neutrophil, and monocytes) can be observed with intracytoplasmic merozoites approximately 4-6μm in diameter often displacing the host nuclei.1,3,4 On cytologic smear intracytoplasmic gamonts can be rarely observed in monocytes.1
This case is an important reminder of the limitations of PCR as a confirmatory or definitive diagnostic tool. Despite both historic Hepatozoonosis and impressive multisystemic disease, the antemortem real time PCR was negative at the time of submission. A false negative PCR indicates that Hepatozoon DNA was not detected in the specific sample submission or may suggest that the numbers of detectable organisms is below the limit of detection (i.e. decreased numbers of organisms following treatment, a chronic carrier state, or the occurrence of new strain variations). When possible, PCR should be utilized in conjunction with corroborating tests such as cytologic blood smears, fine needle aspirates of hematopoietic and lymphoid tissue, and indirect fluorescent antibody (IFA). Muscle biopsy and/or necropsy remain the gold standard for definitive diagnosis.1
Contributing Institution:
North Carolina State University College of Veterinary Medicine
https://cvm.ncsu.edu/php/
JPC Diagnosis:
- Heart: Myocarditis, histiocytic and lymphoplasmacytic, multifocal, moderate with intracellular apicomplexan meronts.
- Lymph node: Sinus histiocytosis, diffuse, mild with apicomplexan meronts.
JPC Comment:
This week’s moderator was Dr. Tony Alves, former JPC Veterinary Pathology Director and current pathologist at the National Institute of Allergy and Infectious Diseases (NIH/NIAID). Given his role within the NIAID Infectious Disease Pathogenesis Section, the theme for this conference was hardly surprising for participants.
This first case was relatively straightforward for residents (who, as WSC tradition provides, were not provided case history). From subgross magnification,, the large meronts (apicomplexan cysts) and inflammatory foci are scattered among the multiple tissues provided, underscoring that these changes contributed to the decline of this animal. The size and lamellation of these meronts along with the concurrent inflammation and hypertrophic osteopathy are highly suggestive of H. americanum over H. canis.1,2 In the latter case, meronts are smaller and rarely incite significant inflammation. Conference participants discussed the cell of origin infected by sporozoites on the slide and concluded that cardiac myocytes and histiocytes (particularly within the lymph node) were most likely in this case. Stains for PAS, PAS-Alcian Blue, and Giemsa all reliably stained the cyst wall, mucinous material, and/or merozoites for this case.
Dr. Alves noted several ancillary features in this case, which might be overlooked at first glance, that hint at a wider clinical story.. Within the heart, there is a solitary arteriole with amorphous eosinophilic material that expands the tunica media and compresses the lumen, consistent with either hyalinosis (an aging change) or amyloidosis given the concurrent inflammation in section. Additionally, there were few megakaryocytes present within the lymph node, suggestive of extramedullary hematopoiesis in response to anemia from hemoabdomen.
Conference participants discussed several ruleouts for tissue cysts. Trypanosoma cruzi and Sarcocystis spp. both form discrete tissue cysts. In contrast to Hepatozoon (particularly H. americanum) these cysts lack lamellation (Sarcocystis and Trypanosoma); size and shape is variable and may appear similar. Distribution to cardiac myocytes is common among all 3 species, though distribution to other tissues (e.g. lymph node) may be a distinguishing diagnostic feature absent IHC. Additionally, smaller tissue cysts to consider are Toxoplasma and Neospora. We recently covered T. cruzi in WSC 2024-2025 (Conference 3, Case 3). For another example of H. americanum from the WSC archives, see Case 2, Conference 18, 2015-2016.
References:
- Baneth G, Allen K. Hepatozoonosis of Dogs and Cats. Veterinary Clinics of North America. 2022; 52(6):1341-1358.
- Craig LE, Dittmer KE, Thompson KG. Bones and Joints. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Louis, MO: Elsevier; 2016: 94
- Cummings CA, Panciera RJ, Kocan KM, Mathew JS, Ewing SA. Characterization of stages of Hepatozoon americanum and parasitized canine host cells. Vet Pathol. 2005;42(6):788-796.
- Ewing SA, Panciera RJ. American canine hepatozoonosis. Clinical Microbiology Reviews. 2003; 16(4):688-97.
- Panciera RJ, Mathew JS, Ewing SA, Cummings CA, Drost WT, Kocan AA. Skeletal Lesions of Canine Hepatozoonosis Caused by Hepatozoon americanum. Veterinary Pathology. 2000;37(3):225-230.
- Parkins ND, Stokes JV, Gavron NA, Frankovich AN, Varela-Stokes, JV. Scarcity of Hepatozoon americanum in Gulf Coast tick vectors and potential for cultivating the protozoan. Vet Parasitol. 2020
- Potter TM, Macintire DK. Hepatozoon americanum: an emerging disease in the south-central/southeastern United States. Journal of Veterinary Emergency and Critical Care. 2010; 20:70-76.
- Valli VEO, Kiupel M, Bienzle D. Hematopoietic system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals. 6th ed. Vol 3. St. Louis, MO: Elsevier; 2016:109-111.
- Van Vleet JF, Valentine BA. Muscle and tendons. Hepatozoonosis. In Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 1. Elsevier Saunders; 2007: 240.