Signalment:
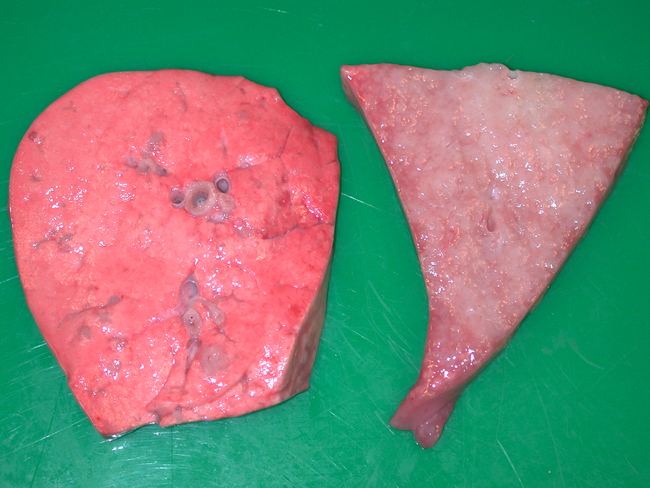
Gross Description:
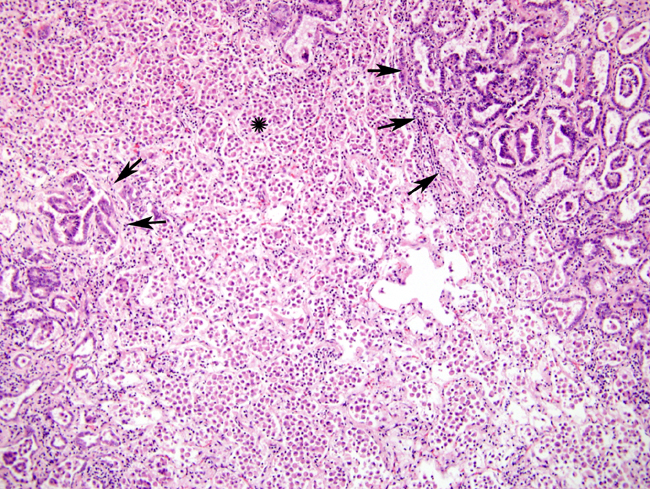
Histopathologic Description:
Morphologic Diagnosis:
1. Ovine pulmonary adenocarcinoma (OPA)
2. Severe, diffuse, histiocytic interstitial pneumonia with marked BALT hyperplasia
Condition:
Contributor Comment:
In this case, the gross changes were typical of the classical form of the disease. An atypical form occurs (although has apparently not been reported in Scotland) whereby the nodules are more discrete, harder and much drier. Lesions generally only occur in the lungs although metastasis to lymph nodes can arise and is one of the main features helping to classify the lung lesions as truly neoplastic, rather than simply proliferative. Extrathoracic metastases have also been reported4. Electron microscopy has confirmed that the alveolar proliferations are composed of type II pneumocytes, while those arising in bronchioles are composed of Clara cells. Both cell types are secretory, accounting for the copious amounts of frothy fluid produced, which tends to flood the respiratory passages in the classical form. This excessive fluid accumulation is absent from the atypical form6. Microscopically, the neoplasm is classified as a bronchioloalveolar carcinoma. The histological findings in this case were typical and tend to be identical between the two forms4.
The lung tropism of the JSRV and resultant neoplastic transformation of pulmonary epithelial cells is apparently unique in the retrovirus group. The exact mechanism of neoplastic transformation is the subject of much current research. Two genes appear to be important to this tropism and viral infectivity, the env gene and the long terminal repeat (LTR) gene. The env gene permits viral entry of cells because it encodes the viral glycoprotein which allows interaction with cell receptors. Thus, the virus can only infect cells which specifically express its receptor, although pulmonary epithelial cells are not the only cells to do so4. The LTR gene is integrated into the cellular genome after viral entry and induces viral expression by interacting with cellular transcription factors. It can activate proto-oncogenes via insertional mutagenesis, whereby provirus is inserted near a proto-oncogene and drives its overexpression.Â
Of these two genes, the env gene is gaining favor as the more likely oncogenic agent since it has been shown to function as an oncogene, at least experimentally in mammalian fibroblast and epithelial cell lines. The mechanism of cell transformation is unclear although it is believed to involve the cytoplasmic tail of the envelope transmembrane protein as well as two downstream cell signaling pathways, H/N-Ras-MEK-MAPK and Akt-mTOR2. The insertional mutagenesis theory seems less likely since the random nature and the infrequency of insertion in the desired place within the genome would not be efficient enough for proto-oncogene activation4.
The sheep genome also contains 15-20 copies of endogenous retrovirus which is very similar to the exogenous JSRV. The main difference lies in the LTR region of the genome such that the endogenous form of the virus does not have the same transcriptional efficiency in pulmonary epithelial cells as the exogenous, tumor-inducing form. The existence of the endogenous virus may explain the lack of an antibody response in OPA infected sheep, since the endogenous elements may promote immunotolerance during fetal development4.
There was marked BALT hyperplasia in this case, for which there could have been two main reasons. Firstly, it can occur in the atypical form of OPA. We felt this was less likely since the concomitant fibrosis and marked lymphoplasmacytic inflammation usually seen in the atypical form were not present6. Secondly, the possibility of concurrent maedi was considered since combined infections have been frequently recognized; no further testing was performed to confirm or refute this possibility5. There was also quite severe histiocytic inflammation. The widespread and marked infiltrate of plump macrophages is commonly found around neoplastic nodules; they are believed to be induced by the excessive surfactant protein production but their exact role in the pathogenesis is still unclear. Recent work suggests they reflect a cellular immune response to the presence of neoplastic cells. The apparent ineffectiveness of this response is believed to be due to putative immunosuppressive properties of the excess surfactant protein7.Â
JPC Diagnosis:
2. Lung: Lymphofollicular hyperplasia, diffuse, moderate.
3. Lung: Pneumonia, interstitial, multifocal, mild.
Conference Comment:
References:
2. Caswell JL, Williams KJ: Respiratory System. In: Jubb, Kennedy, and Palmers Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 2, pp. 621-622. Elsevier Limited, St. Louis, MO, 2007
3. Maeda N, Fu W, Ortin A, de las Heras M, Fan H: Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines. J Virol 79:4440-4450, 2005
4. Palmarini M, Fan H: Retrovirus-induced Ovine Pulmonary Adenocarcinoma, an Animal Model for Lung Cancer. J Natl Cancer Inst 93:1603-1614, 2001
5. Rosadio RH, Sharp JM, Lairmore MD, Dahlberg JE, DeMartini JC: Lesions and retroviruses associated with naturally occurring ovine pulmonary carcinoma (sheep pulmonary adenomatosis). Vet Pathol 25:58-66, 1988
6. Salvatori D, De las Heras M, Sharp M: Ovine pulmonary adenocarcinoma: the story to date. In Practice 4:2004
7. Summers C, Norval M, De las Heras M, Gonzalez L, Sharp JM, Woods GM: An influx of macrophages is the predominant local immune response in ovine pulmonary adenocarcinoma. Vet Immunol Immunopathol 106:285- 294, 2005