Signalment:
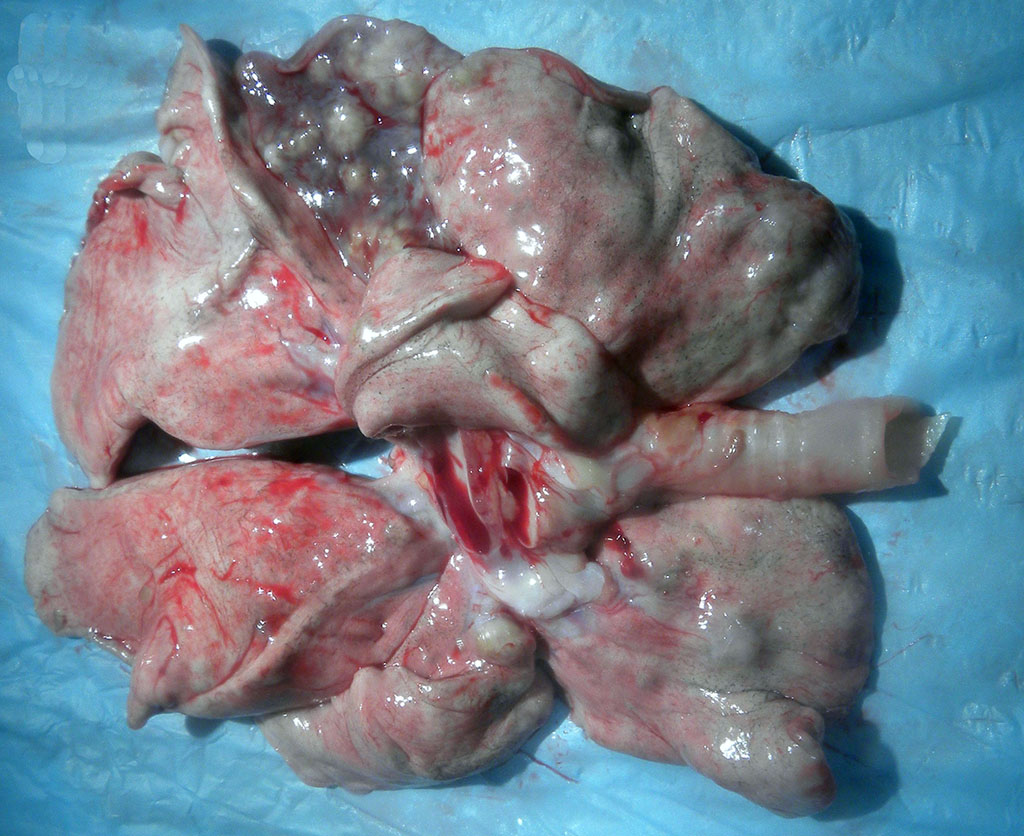
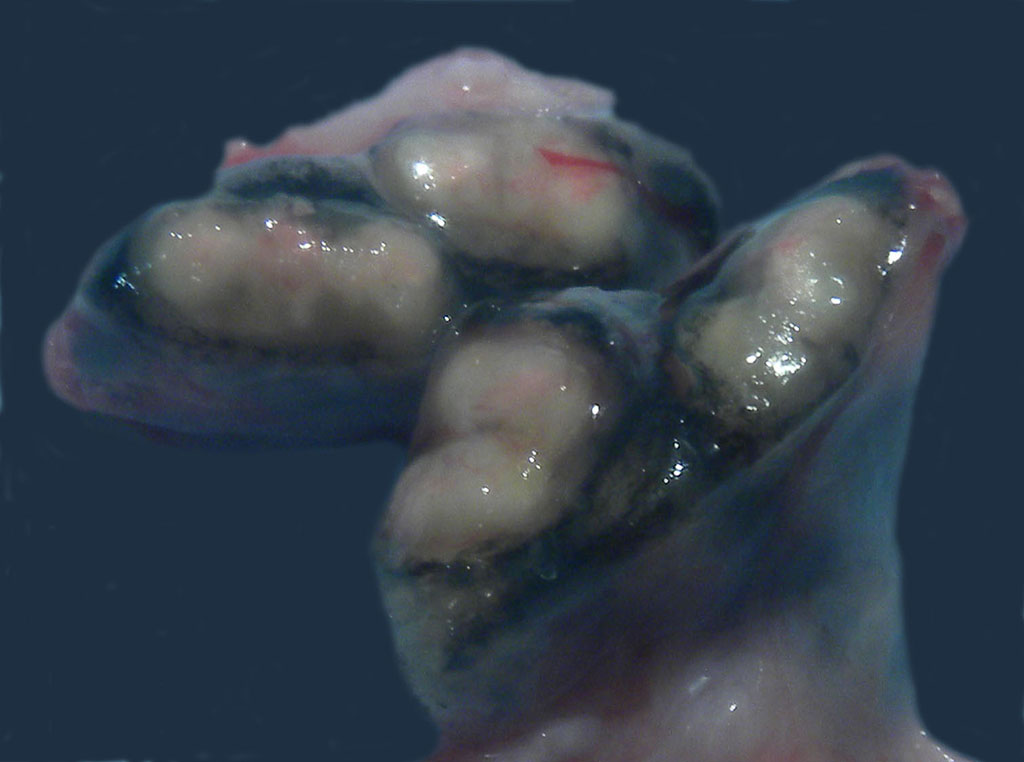
Gross Description:
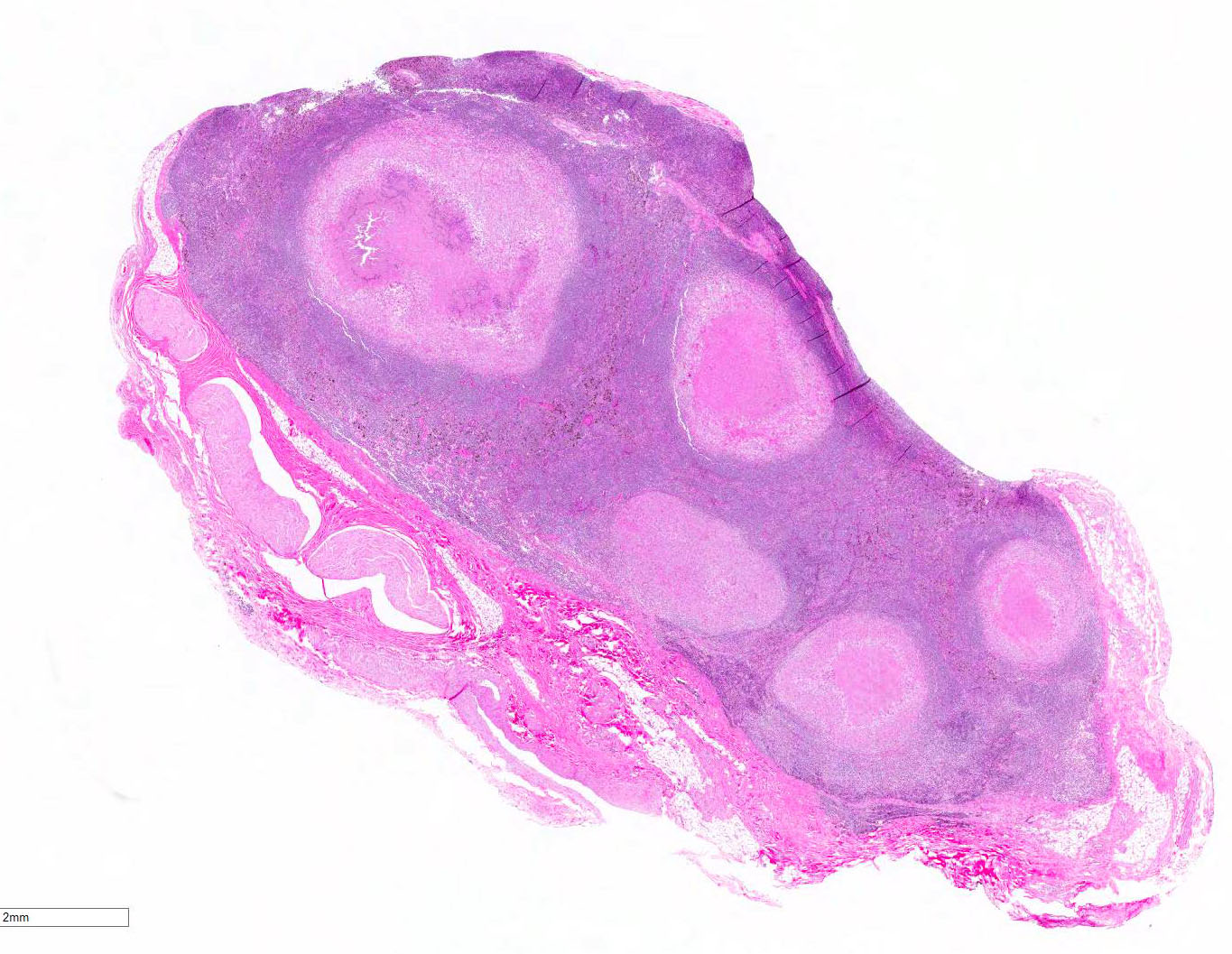
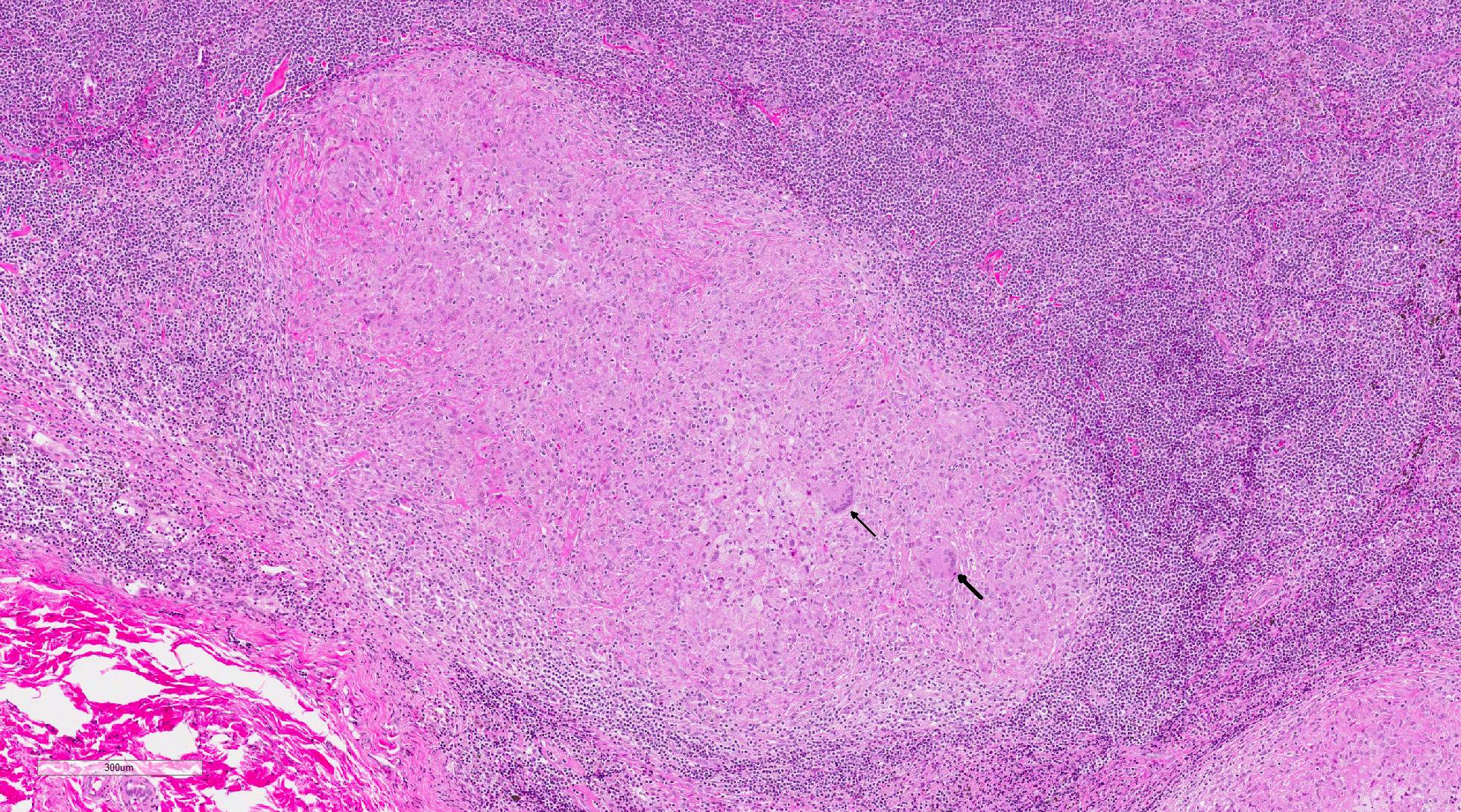
Histopathologic Description:
Morphologic Diagnosis:
Relevant microscopic findings of tissues not submitted: Similar focus of necro-granulomatous inflammation containing rare intracellular acid-fast bacilli in epithelioid macrophages and multi-nucleated giant cells was present in the lung.
Lab Results:
|
Grade |
Score |
Description |
|
0 |
Negative |
No reaction |
|
1 |
Negative |
Bruise |
|
2 |
Negative |
Erythema, no swelling |
|
3 |
Suspect |
Erythema with minimal swelling OR slight swelling without erythema |
|
4 |
Positive |
Obvious swelling with eyelid droop and erythema |
|
5 |
Positive |
Swelling and/or necrosis with eyelid closed |
Table 2: Tuberculosis testing
|
Date |
Test |
Result |
|
12-14-09 |
TST MOT |
Grade 0 (negative) |
|
12-28-09 |
TST MOT |
Grade 0 (negative) |
|
01-05-10 |
Thoracic radiographs |
unremarkable |
|
01-11-10 |
TST MOT |
Grade 0 (negative) |
|
11-05-10 |
TST MOT |
Grade 3 (suspect) |
|
11-08-10 |
TST MOT |
Grade 0 (negative) |
|
11-08-10 |
PRIMAGAM® |
Negative |
|
11-08-10 |
PrimaTB STAT-PAK® |
Negative |
|
04-08-11 |
PrimaTB STAT-PAK® |
Negative |
|
04-14-11 |
PrimaTB STAT-PAK® |
Negative |
Condition:
Contributor Comment:
Mycobacterium tuberculosis (Mtb) was cultured from the tracheobronchial lymph node by the National Veterinary Services Laboratory (USDA, APHIS) in this case. Mtb was isolated from lung and/or tracheobronchial lymph node in 7 of 12 animals involved in the outbreak. Genotyping was performed and all isolates shared the same spoligotype and VNTR-11 profile, confirming a common source of infection. The results of tuberculosis testing on this animal from the time of acquisition to death appear in Table 2.
Mtb is an aerobic, nonmotile, nonsporulating, straight to slightly curved 1-4 micron rod-shaped bacillus from the order Actinomycetales. Mtb is a member of the M. tuberculosis complex, which includes other Mycobacterium species that induce tubercle formation including M. bovis and its variants M. caprae and M. pinnipedi, and less frequently identified species M. africanum, M. canetti, and M. microti.10 The bacterium features a thick, waxy cell wall containing mycolic acids which impart unique characteristics including acid-fast staining positivity and, along with other cell wall components, resistance to environmental desiccation, antibiotics, and phagocyte destruction.
In macaques, infection occurs following inhalation of aerosolized bacteria into the lungs.7 Ingestion is an infrequently encountered route of inoculation. Resident tissue macrophages phagocytize bacteria, and through multiple mechanisms, bacteria prevent lysosomal degradation and subsequently replicate within an intracytoplasmic phagosome.10 Dendritic macrophages may then disseminate bacilli to draining lymph nodes and distant organs. Complex biochemical signaling and immune cell activation pathways, especially of CD4+ and CD8+ T cells, are required for control of primary infection.2,4,7,9,10 The latent, non-infectious stage ensues when bacteria have been sequestered within granulomas characterized by a central accumulation of necrotic debris that is rarely mineralized surrounded by infected epithelioid macrophages and Langhans multinucleated giant cells, lymphocytes, and a fibrous capsule.10 Bacteria persist within the tubercle and may reactivate, typically in association with immunosuppression.5,9 Typical tubercles can be found in any organ and most commonly disseminate from the lungs and hilar lymph nodes to the liver, spleen, kidney, vertebra, and gastrointestinal tract.
Cases of spontaneous and experimental tuberculosis are reported in several primate species with variation in susceptibility to disease.1,2,4,7,9 Macaques are known to be highly susceptible and present with a disease course of fulminate primary, active-chronic, or latent infection with or without reactivation. Active infection and bacterial shedding can occur over weeks to months, during which time clinical signs may be vague, weight loss and cough, or inapparent.1,6,9 Intermittent bacterial shedding in latently infected macaques has been documented experimentally, evidenced by sporadic positive bacterial culture of gastric and bronchial alveolar lavage fluids.9 This finding suggests that disease occurs on a continuum between active and latent infection. Cynomolgus macaques are thought to be relatively more resistant to clinical disease than rhesus macaques, and in experimental studies, 40-50% developed latency.2,6,9 Spontaneous reactivation of latent infection in the absence of experimental immunosuppression is infrequent, reportedly less than 5% in cynomolgus macaques.5
Mtb is an important zoonotic pathogen with potentially devastating effects in captive macaque populations and routine measures have been adopted by US facilities for screening of imported and domestically-reared animals. The most commonly utilized screening test is the tuberculin skin test (TST).8 MOT, a combination of Mycobacterium antigens from multiple species, is injected intradermally, typically into the upper eyelid.8 The site is checked for reaction at 24, 48, and 72 hours post-injection (see Table 1 for grading).8 In affected animals, a delayed-type hypersensitivity reaction to antigen produces swelling, erythema, and/or necrosis at the injection site. False negatives may occur in animals with anergy and disseminated disease, and in animals that are in the latent stage of infection.8 The MOT antigen is not specific for M. tuberculosis and M. bovis, and false positives may occur in the face of infection with a non-tubercular Myco-bacterium species. The TST is the only approved screening test for internationally acquired animals undergoing US quarantine, and three negative tests two weeks apart are the standard requirement.8 Typically, animals that have cleared international quarantine and domestically reared animals are tested on a semiannual basis.
Alternative commercial tests utilized during this outbreak included the whole-blood interferon gamma (INFγ) test PRIMAGAM® (Prionics USA Inc., La Vista, NE) and the lateral flow immunoassay PrimaTB STAT-PAK® (CHEMBIO).8 In the PRIMAGAM® test, the concentration of INFγ is measured following incubation with purified protein derivative (PPD) of M. bovis (bPPD) and M. avium (aPPD).8 Positive tests that yield a higher reaction to bPPD vs. aPPD indicate sensitization to antigens from either M. tuberculosis or M. bovis, while a higher aPPD suggests sensitization to M. avium and retesting after a 2-week period is recommended.7 The PrimaTB STAT-PAK® test detects antibody response to three TB-specific antigens (ESTAT-6, CFP-10, MPB83).8 Serum, whole blood, or plasma can be used.
During this outbreak, the first animal was initially detected by routine tuberculosis surveillance with a positive TST. Affected animals exhibited variable responses to TST, PRIMAGAM®, and PrimaTB STAT-PAK® testing performed during the outbreak. Five months prior to the outbreak this animal exhibited a grade 3 reaction with a TST. A follow-up TST on the contralateral eyelid and PRIMAGAM® and PrimaTB STAT-PAK® tests were all negative. Likewise, all three tests performed at the time of the outbreak were negative. The gold-standard for diagnosis of M. tuberculosis is culture, which was positive for this animal. This outbreak is thought to have occurred as a result of reactivation and shedding from a latently infected animal in the room. The source was not identified. This outbreak illustrates the challenges of antemortem diagnosis and underscores the importance of routine tuberculosis screening as clinical signs were not observed in any of the animals in this cohort.
JPC Diagnosis:
Conference Comment:
Infection is typically via inhalation of aerosol droplets from an infected individual with pulmonary disease. Humans are the only known natural reservoir for Mtb. The bacilli then travel to the alveoli where they are phagocytized by alveolar macrophages. The ability of the organism to survive within macrophages is the key to its pathogenicity.10 Once inside the macrophage, bacilli can survive and replicate by inhibiting the fusion of the phagolysosome, thus preventing oxygen radical formation and avoiding proteolytic enzymes produced by the host macrophage. Natural resistance-associated macrophage protein (NRAMP) is a membrane protein that is involved in innate immunity to infection by M. bovis in humans and mice.1,3,10
Once exposed to the bacteria, alveolar macrophages are stimulated by ligation of Toll-like receptor 2 (TLR2) and TLR4 to secrete pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1) IL-6 and IL-12, in addition to a variety of C-C chemokines to recruit leukocytes to the site of infection. Alveolar macrophages also present phagocytized mycobacterial antigen to naïve T cells via major histocompatibility complex class II (MHC II).3,10 This binding to the T-cell receptor and exposure to IL-12 secretion stimulates T cell differentiation to CD4+ T helper 1 (Th1) lymphocytes. These Th1 lymphocytes then secrete interferon gamma (IFN-gamma) and IL-2 stimulating the classical activation of macrophages, favoring a cell mediated immune response. TNF-alpha and IFN-gamma work synergistically to form tuberculoid granulomas in an effort to prevent the spread of the infection to other sites in the body and transmission of the infection to other animals.3,10 This granuloma is maintained by a type IV (delayed-type) hypersensitivity (DTH) reaction to persistent antigenic stimulation due to Mtbs ability to survive within macrophages and extracellularly within the granuloma. Ongoing infection eventually leads to caseous necrosis within central area of the granulomas. Interestingly, bacilli can survive for years within the caseous centers and be reactivated by immunosuppression, malnutrition, or concurrent infection.1,3,10
Additionally, dendritic cells in the lung also phagocytize Mtb by binding receptor DC-SIGN via the bacterial cell wall virulence factors lipoarabinomannan mannose-capped (ManLAM), complement receptor 2 (CR3), and mannose receptor (MR) and then migrate to regional lymph nodes to present mycobacterial antigens to lymphocytes. Spread from the lungs to the tracheobronchial may lymph node may explain the location of the lesion within the tracheobronchial lymph node, in this case.3,10
References: