Wednesday Slide Conference 2022-2023
Conference 15
Case II:
Signalment:
Three ~12 week old male mice (Mus musculus) on a C57BL/6J background were evaluated.
History:
Mice were heterozygous knockouts for a gene involved in tRNA modification* and
had no clinically detectable phenotype. The mutation had been generated in C57BL/6J mice (B6J) and backcrossed to wild-type B6J.
*The phenotype of this mutation is as yet unpublished and permission has not been granted to reveal the specific gene.
Gross Pathology:
Two of the three male mice had small livers at necropsy.
Laboratory Results:
No laboratory findings reported.
Microscopic Description:
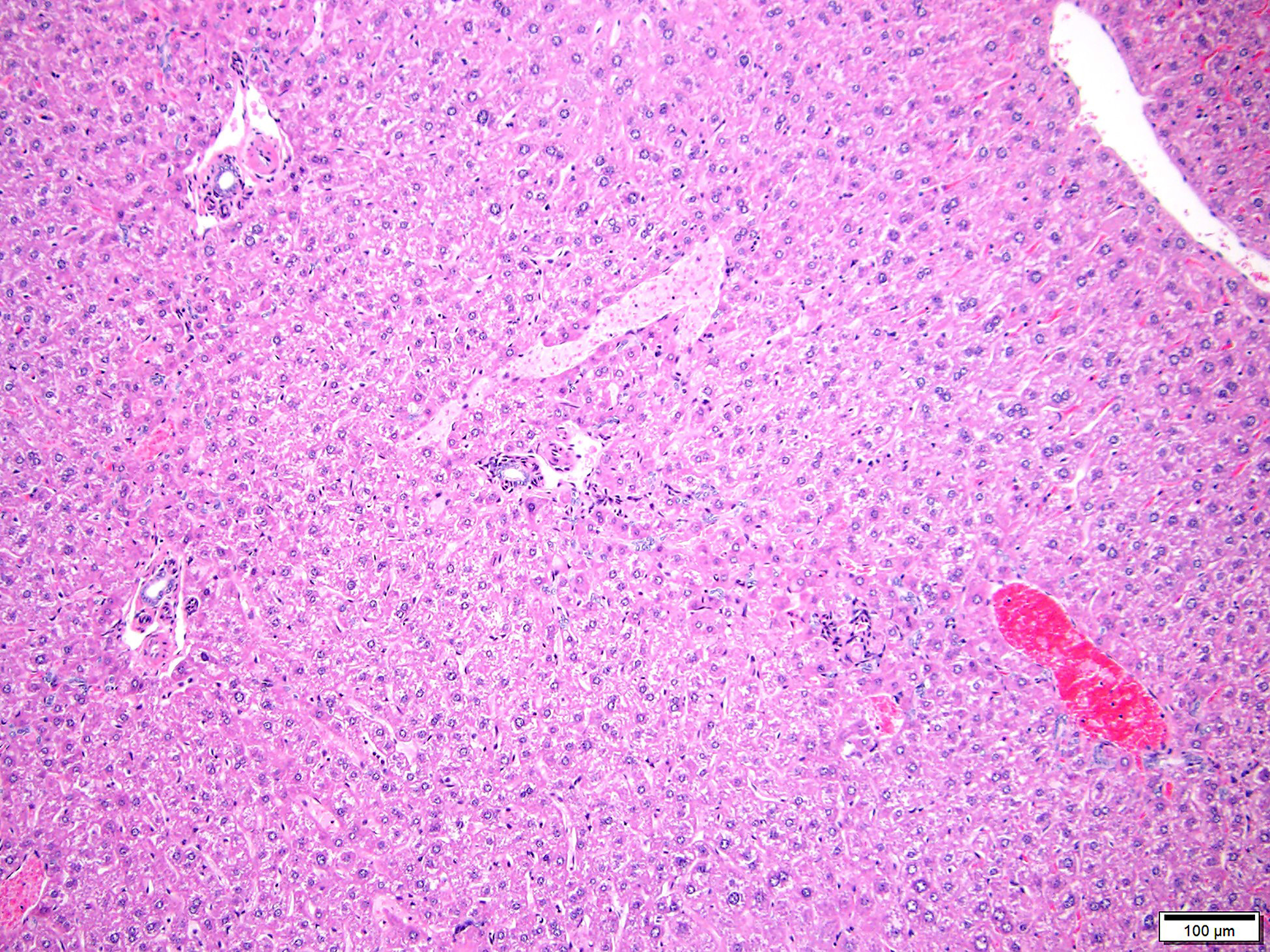
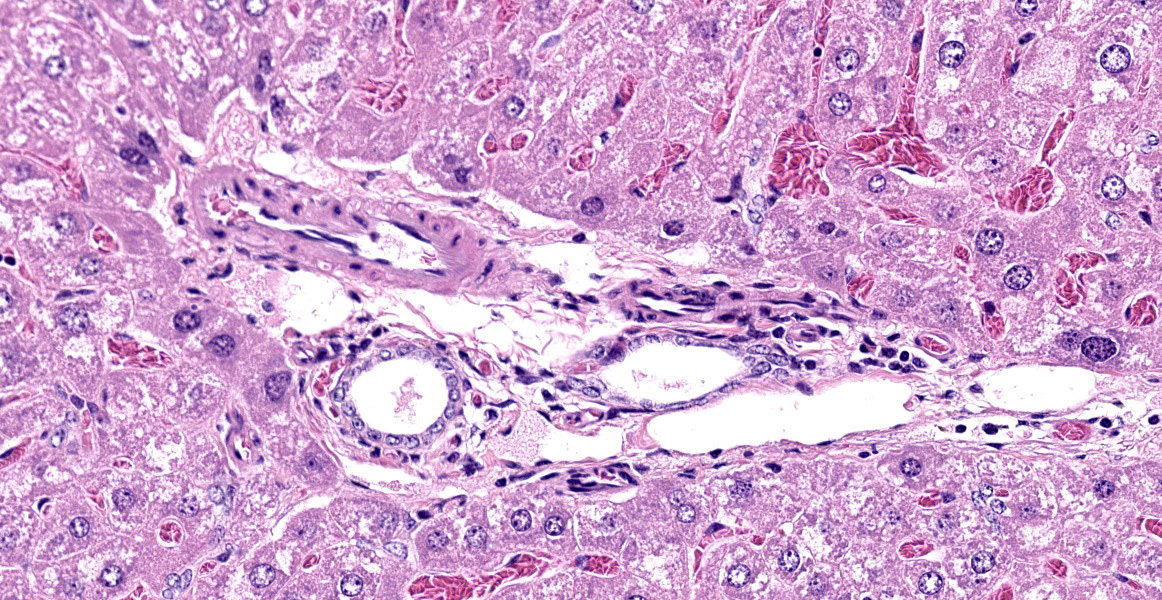
In the livers of all three mice, portal veins were diffusely absent or small and slit-like. Portal triads frequently contained multiple hepatic arteriolar profiles (arteriolar reduplication), many of which had thickening of the tunica media. Periportal lymphatics were dilated and prominent and mild dilation of hepatic sinusoids was also present multifocally. Two of the three mice also had multifocal, random and perivascular small aggregates of infiltrating leukocytes, sometimes accompanied by focal loss or degeneration of hepatocytes. Infiltrating leukocytes consisted of mononuclear cells and, less frequently, neutrophils.
Contributor?s Morphologic Diagnoses:
Liver:
- Portal vein hypoplasia, diffuse, severe, with lymphatic ectasia and arteriolar reduplication
- Mononuclear and neutrophilic infiltration, random and perivascular, mild
Contributor?s Comment:
Portal vein hypoplasia is the histologic manifestation of intra or extrahepatic portosystemic shunting (PSS).1,4 In dogs, the term microvascular dysplasia (MVD) has been used to describe histologic findings of PSS in the absence of a detectable shunt but, because the histology and pathogenesis of MVD overlap with that of congenital PSS, the morphologic diagnosis of portal hypoplasia is now preferred for both conditions according to the World Small Animal Veterinary Association.4 Portal vein hypoplasia is characterized by absent or slit-like portal vein profiles and increased hepatic arteriolar profiles, or arteriolar reduplication. Arteriolar reduplication can occur with portal hypertension6, however congenital PSS lacks portal hypertension and, as in this submission, associated changes of portal fibrosis may be absent. In PSS, arteriolar duplication may represent a compensatory response to decreased portal delivery of trophic factors to hepatocytes.1
Spontaneous congenital portosystemic shunting (PSS) with a microscopic appearance similar to this submission was recently described in a fairly high (~25%) number of C57BL6/J mice.2 Affected mice were both transgenic and wild-type and originated from multiple different institutions, thus the finding was believed to be background strain-related. Shunting was not visible grossly and was confirmed by microscopy and by specialized imaging of blood flow through the liver. Based on shunt location (within the left side of the liver), pathogenesis was speculated to involve persistence of the ductus venosus (shunts blood from placenta to vena cava in the fetus). Inheritance was non-Mendelian and epigenetic alterations were suspected. As is typical of PSS in dogs, bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) remained within historic reference intervals, although affected mice had greater variation in AST and ALT. Bile acids may be more diagnostic but are not routinely performed in mice. Of note, affected mice were originally identified by an abnormal brain neurochemical profile (elevated glutamine) during screening by proton magnetic resonance spectroscopy. Glutamine is an end-product of ammonia detoxification by astrocytes in hepatic encephalopathy. Although symptoms of hepatic encephalopathy were not described, elevation of brain glutamine levels correlated with portosystemic shunting and may indicate some level of subclinical metabolic encephalopathy.2 Thus, altered metabolism and/or neurochemistry in B6J mice with this defect may affect their suitability for research.
Based on this previous report, intrahepatic shunting may be more common than typically realized in mice and caution is warranted in specifically ascribing a finding of portal hypoplasia to genetic manipulation. In this submission, it was not possible to determine whether portal vein hypoplasia was related to the knockout gene, but spontaneous occurrence was suspected due to the background strain and the lack of a biologically plausible link between the knockout to the lesion. It is intriguing that the lesion was present in 3 of 3 male but 0 of 3 female heterozygotes, however the knockout was not restricted to the liver nor was a sex-linked or liver-restricted phenotype expected. Other mice from the strain were not available for evaluation. No clinical signs suggestive of symptomatic portosystemic shunting (neurological symptoms, small size) were seen, although 2 of the male mice were noted to have small livers at necropsy.
Small foci of random or perivascular infiltrating leukocytes as seen in these mice are common murine background findings. Since they were occasionally accompanied by hepatocyte loss, they may have been exacerbated in this case by decreased trophic supply of nutrients to hepatocytes due to portal hypoplasia.
Contributing Institution:
University of Michigan Unit for Laboratory Animal Medicine In Vivo Animal Core (IVAC) https://animalcare.umich.edu/business-services/vivo-animal-core
JPC Diagnosis:
Liver, portal areas: Venous hypoplasia, multifocal.
JPC Comment:
The histologic appearance in this case is characteristic of portal vein hypoperfusion: decreased portal vein profiles, increased numbers of arteriolar profiles due compensatory hyperperfusion, and hepatocellular atrophy with irregularly spaced small portal triads. Portal vein hypoperfusion can also feature periportal fibrosis, biliary ductular reaction, and lipogranulomas.3,4
Portal vein hypoperfusion is the non-specific result of several distinct diseases. In many of these conditions, hypoperfusion is the result of portal hypertension, which can result in ascites, a useful distinguishing clinical characteristic. Examples of diseases which produce portal hypertension include arterioportal fistulas, obstruction of the portal vein, and primary portal vein hypoplasia.4 Obstruction of the portal vein may occur due to thrombosis or neoplasia and leads to decreased portal blood flow and hypoperfusion. In arteriovenous fistulas, blood travels from a higher-pressure artery into the portal vein, leading to retrograde venous blood flow. While the directly affected lobe contains venous aneurysmal dilations and thick tortuous arteries, subsequent portal hypertension causes characteristic portal vein hypoperfusion in the unaffected lobes. Primary hypoplasia of the portal vein may occur in extrahepatic or intrahepatic locations. Some forms are mild, and histologic lesions are limited to those of hypoperfusion; moderate to severe forms are characterized by portal fibrosis and portal hypertension.
The end result of portal hypertension is acquired portosystemic shunting of blood, where blood flows through numerous enlarged and tortuous collateral veins to reach systemic circulation. Congenital portosystemic shunts, on the other hand, characteristically lack portal hypertension. The flow of blood bypasses the liver, traveling directly from portal vessels to the caudal vena cava or azygous vein and causing portal vein hypoperfusion.
Several features of portosystemic shunts in C57BL/6J mice were described in a recent study evaluating the effect of portal circulation in non-alcoholic fatty liver disease.5 In PSS mice, the hepatic surface was faintly nodular and dull compared to normal livers.5 Staining with pimonidazole, a hypoxia marker, revealed strong staining in the centrolobular region but negative staining in the portal regions, illustrating that arteriolar compensation for portal hypoperfusion was not able to fully oxygenate the distant centrolobular zone.5 When given the hepatotoxin carbon tetrachloride, PSS mice had less hepatic injury than non-PSS mice.5 This was previously reported to be due to decreased CYP2E1 expression; however, this study found CYP2E1 expression was increased in PSS mice and the authors speculated that decreased oxygen availability from hypoxia may have reduced free radical generation.5
The moderator described some of the portosystemic shunts may have on research studies, including smaller livers, altered neurochemical phenotypes, and altered metabolism of drugs and xenobiotics. Participants also discussed the hepatocellular anisokaryosis, particularly in the mid-zonal regions, which is an incidental aging change due to polyploidy.
References:
- Baade S, Aupperle H, Grevel V, Schoon HA. Histopathological and immunohistochemical investigation of hepatic lesions associated with portosystemic shunting in dogs. J Comp Pathol. 2006;134:80-90.
- Cudalbu C, McLin VA, Lei H, et al. The C57BL/6J mouse exhibits sporadic congenital portosystemic shunts. PLoS One. 2013; 8(7): e69782.
- Cullen JM, Stalker MJ. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer?s Pathology of Domestic Animals. Vol 2. 6th ed. St. Louis, MO: Elsevier Ltd; 2016:266-267,289-292
- Cullen JM, van den Ingh TSGAM, Bunch SE, Rothuizen J, Washabau RJ, Desmet VJ: Morphological classification of the circulatory disorders of the canine and feline liver. In: WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. St. Louis, MO: Elsevier. 2006: 41-59, 97-98.
- Meng L, Goto M ,Tanaka H, et al. Decreased Portal Circulation Augments Fibrosis and Ductular Reaction in Nonalcoholic Fatty Liver Disease in Mice. Am J Pathol. 2021; 191(9):1580-1591.
- Nakhleh RE. The pathological differential diagnosis of portal hypertension. Clinical Liver Disease. 2017;10(3):57-62.