WSC 2023-2024, Conference 24, Case 1
Signalment:
28-year-old male American Flamingo (Phoenicopterus ruber).
History:
This flamingo had a 1-year history of progressive weight loss and a 10-year history of progressive bilateral pododermatitis. Two days prior to death, lethargy and separation from the flock were noted and the flamingo was treated with fluids, Excede, and meloxicam.
Gross Pathology:
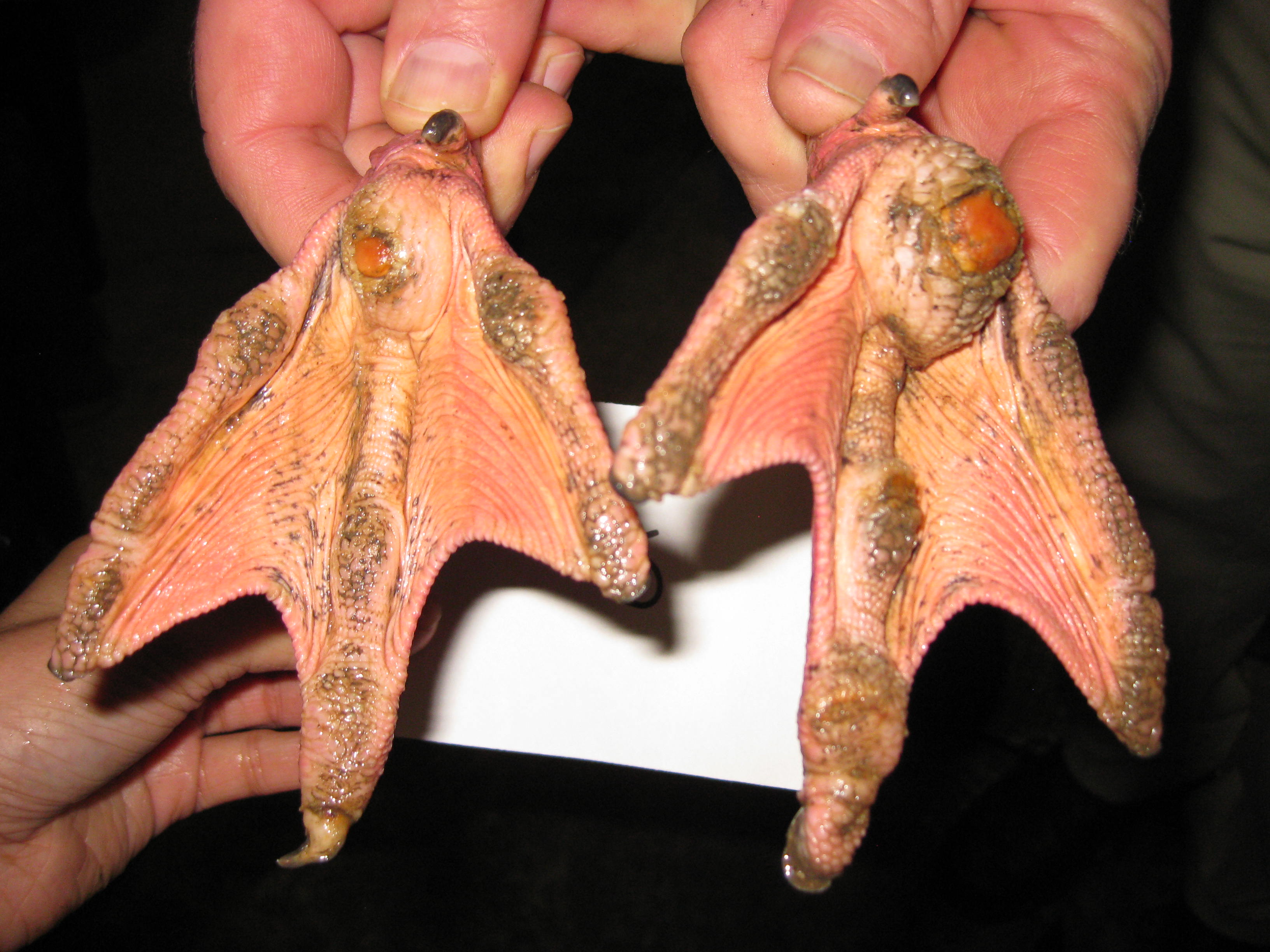
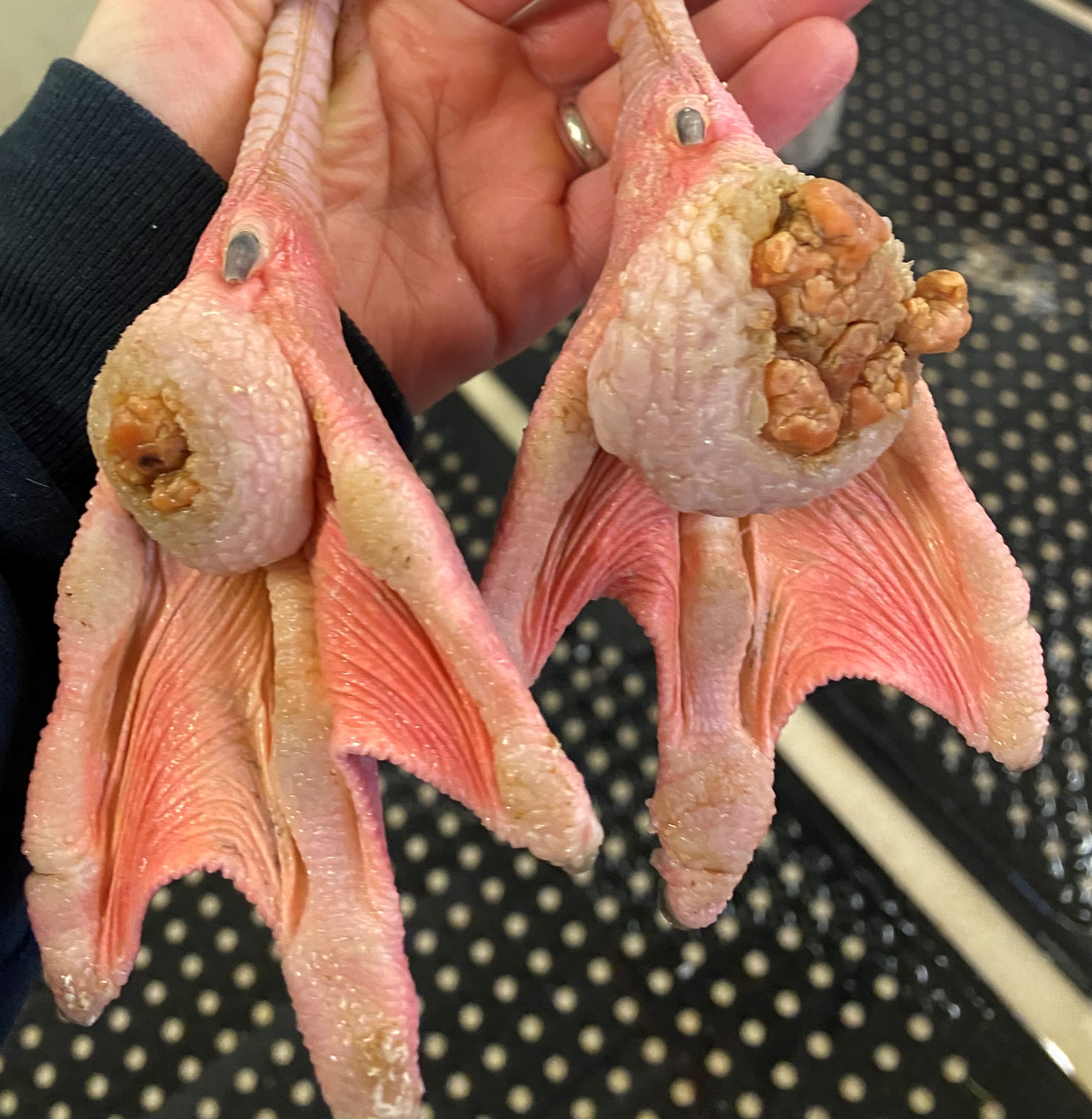
On gross examination, the subcutaneous, coelomic, and pericardial fat stores were significantly reduced (BCS 2/5). Bilaterally, there were round, raised, proliferative nodules measuring 4 cm and 2.5 cm in diameter on the right and left metatarsal pads, respectively. There was a small amount of clear to white mucoid material in the lumen of the glottis. Multifocal, pale gray to brown, flat, circular regions measuring 1-5 mm in diameter were present on the tracheal mucosa. There were white to orange, speckled, granular deposits overlying the pericardium at the level of the right ventricle. Within the ventriculus, there was a 3 mm red, circular, area under the koilin membrane. Throughout all lobes of the liver, there were regions of mild pallor. Both kidneys were markedly enlarged, friable, and
mottled orange to yellow. Within the right stifle, there was a small amount of white, soft, chalky material.
Laboratory Results:
Severely elevated uric acid and heterophilia were present on serum biochemistry panel and complete blood count. Computed tomography revealed bilaterally enlarged kidneys.
Microscopic Description:
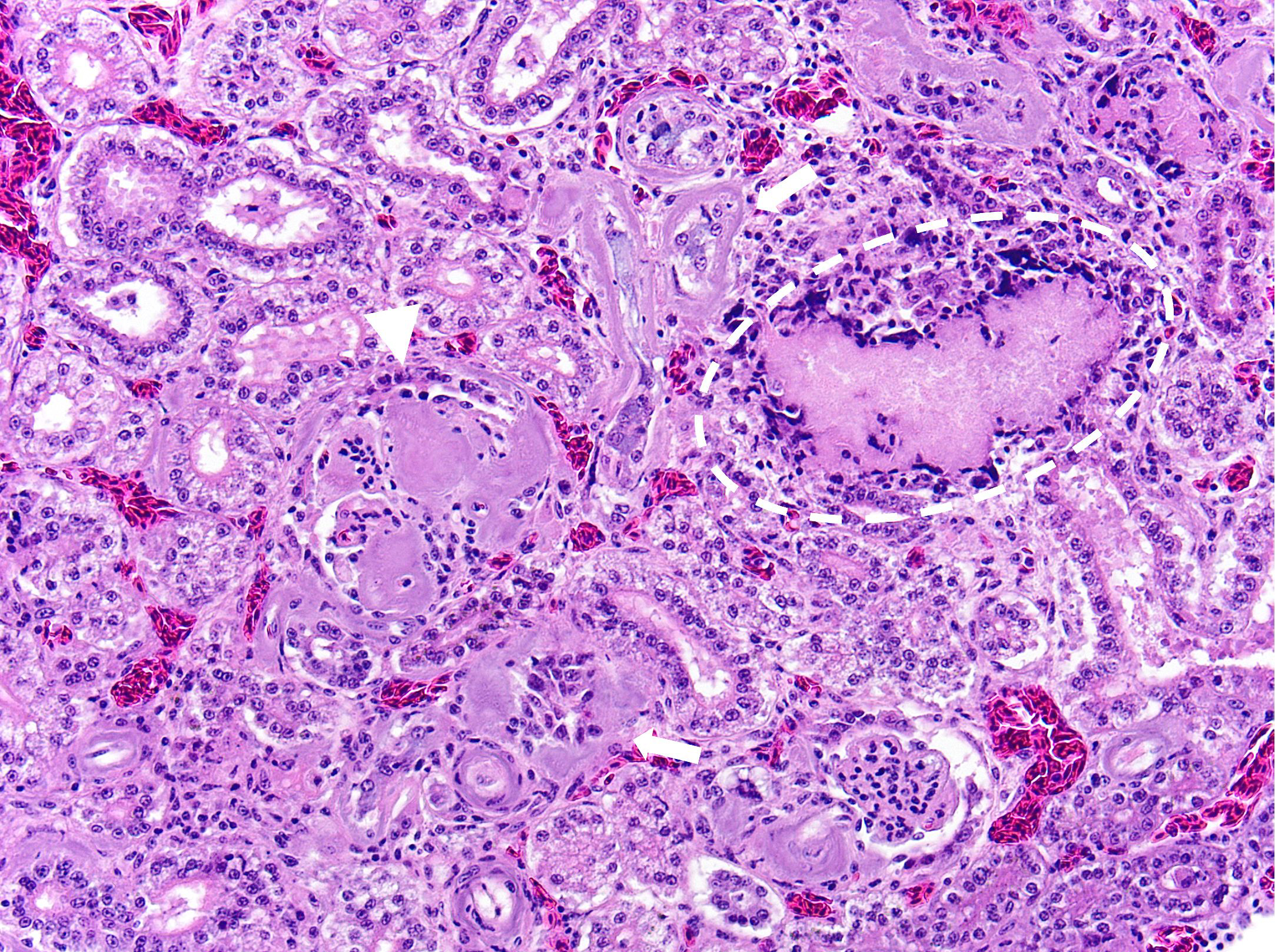
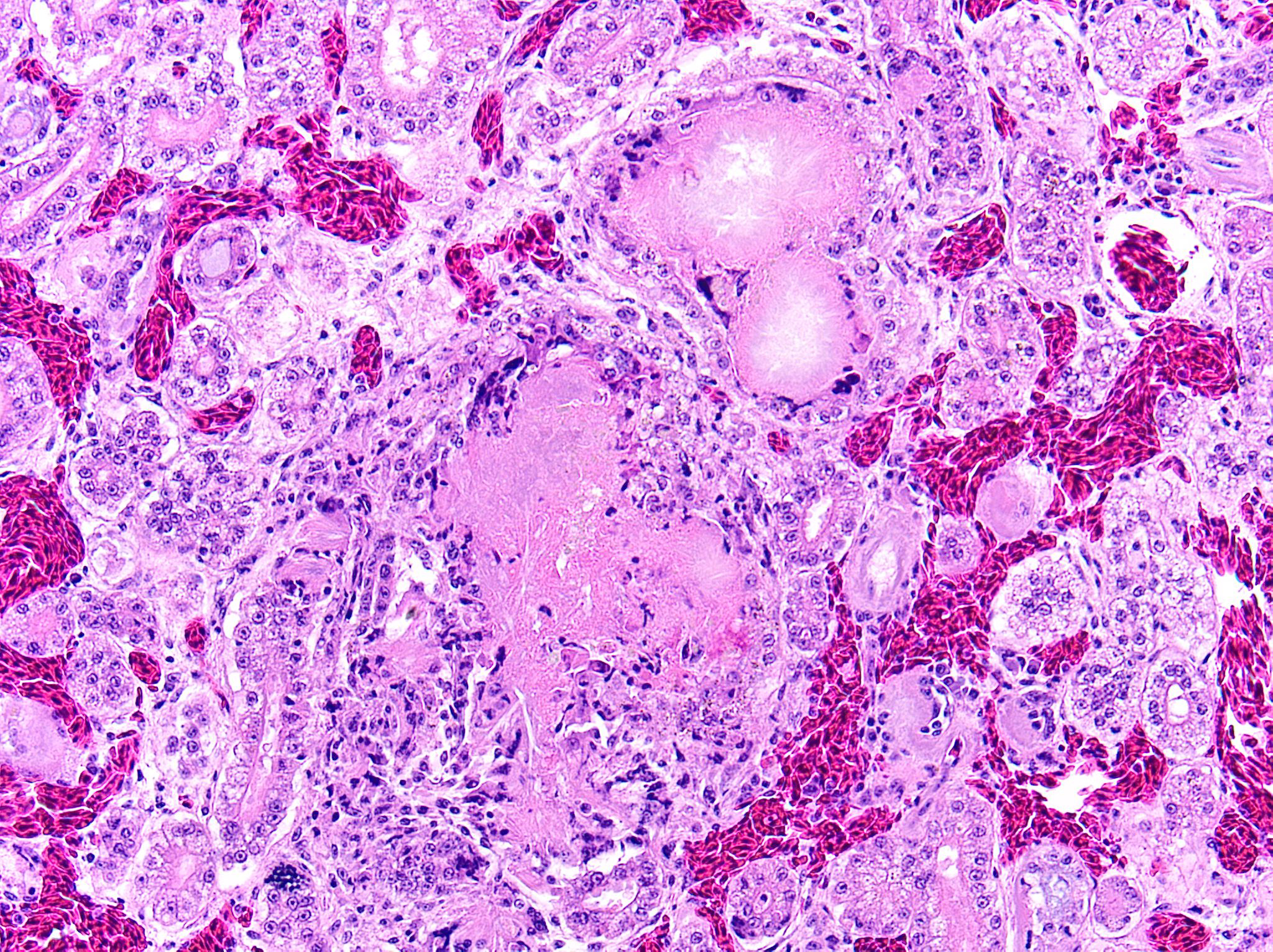
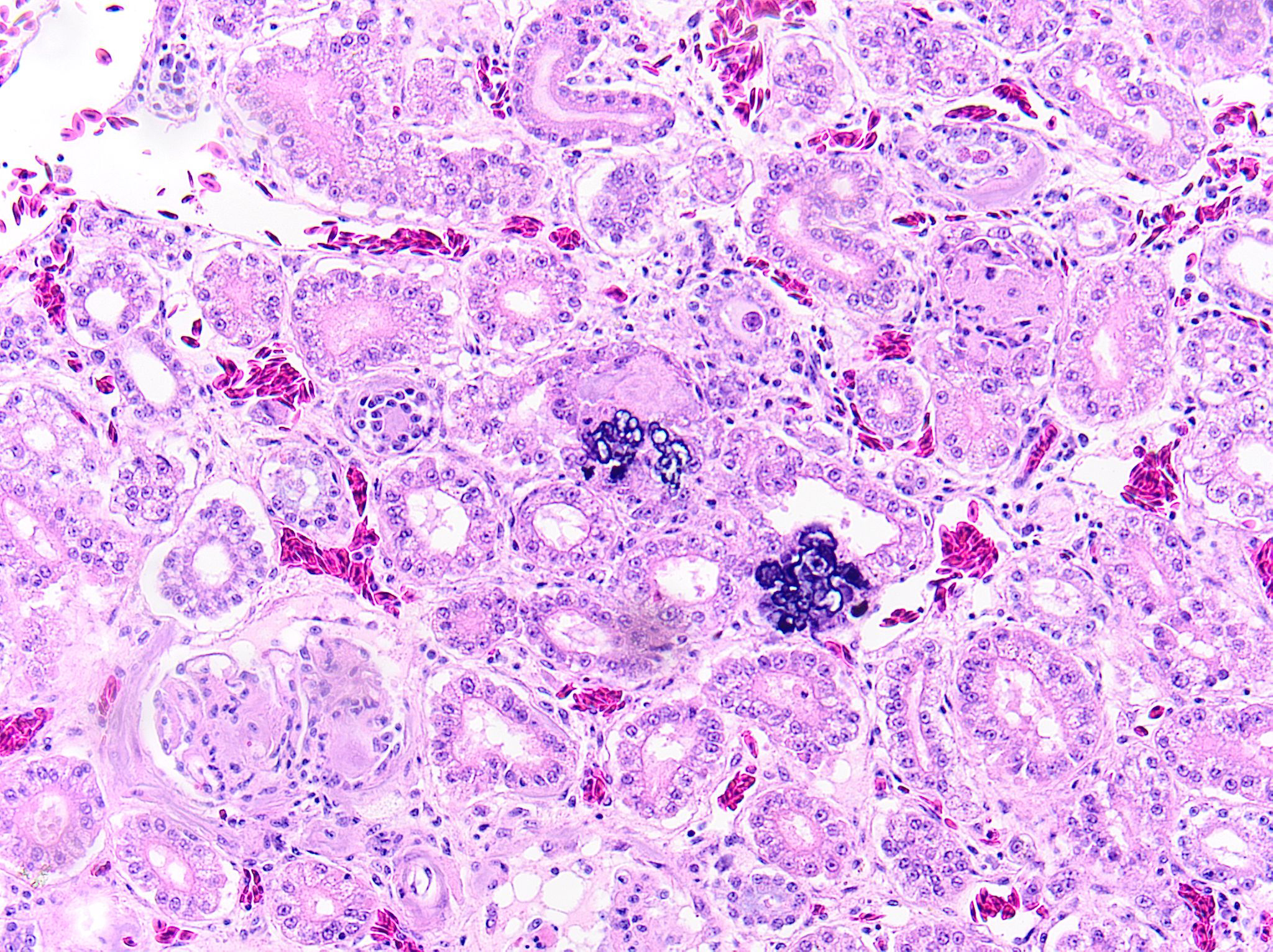
Kidney: Multifocally and randomly expanding and disrupting the interstitium and replacing tubules are foci of brightly eosinophilic cellular and karyorrhectic or pyknotic nuclear debris (necrosis) surrounded by a thin rim of lymphocytes, plasma cells, and histiocytes. These foci measure approximately 150-250 µm and occasionally contain clear clefts within central areas of necrosis (presumed washed-out uric acid crystals). Moderate numbers of red blood cells extend throughout the adjacent interstitium (hemorrhage). Diffusely and globally, glomerular tufts are expanded by variable amounts of acellular, homogenous, eosinophilic, glassy material (amyloid) that compresses capillaries and distorts glomerular architecture. Glomerular tufts are hypocellular with occasional karyorrhectic or pyknotic nuclear debris (necrosis) and frequently adhere to Bowman’s capsule (synechiae). Tubules exhibit one or more of the following changes: swollen epithelium with microvacuolated cytoplasm and large, round vesiculated nuclei (degeneration); hypereosinophilia with pyknotic nuclei (necrosis); increased basophilia with crowded epithelial cells containing hyperchromatic nuclei (regeneration); round, crystalline, deeply basophilic deposits within tubular epithelium (mineral); marked ectasia with flattened, attenuated epithelium and intraluminal eosinophilic to amphophilic proteinaceous fluid or droplets. Multifocally, tubular lumina contain sloughed, necrotic epithelial cells admixed with cellular debris (cellular casts); the basement membranes of affected tubules are often disrupted (tubulorrhexis). Frequently, eosinophilic, homogenous, acellular material (amyloid) expands tubular basement membranes and forms broad cuffs around affected tubules. Multifocally, the tunica media of renal arteries and arterioles is expanded by lightly basophilic to amphophilic amorphous material (amyloid). Mild numbers of lymphocytes and plasma cells expand the renal interstitium.
Great vessels: There are no significant microscopic findings in the great vessels.
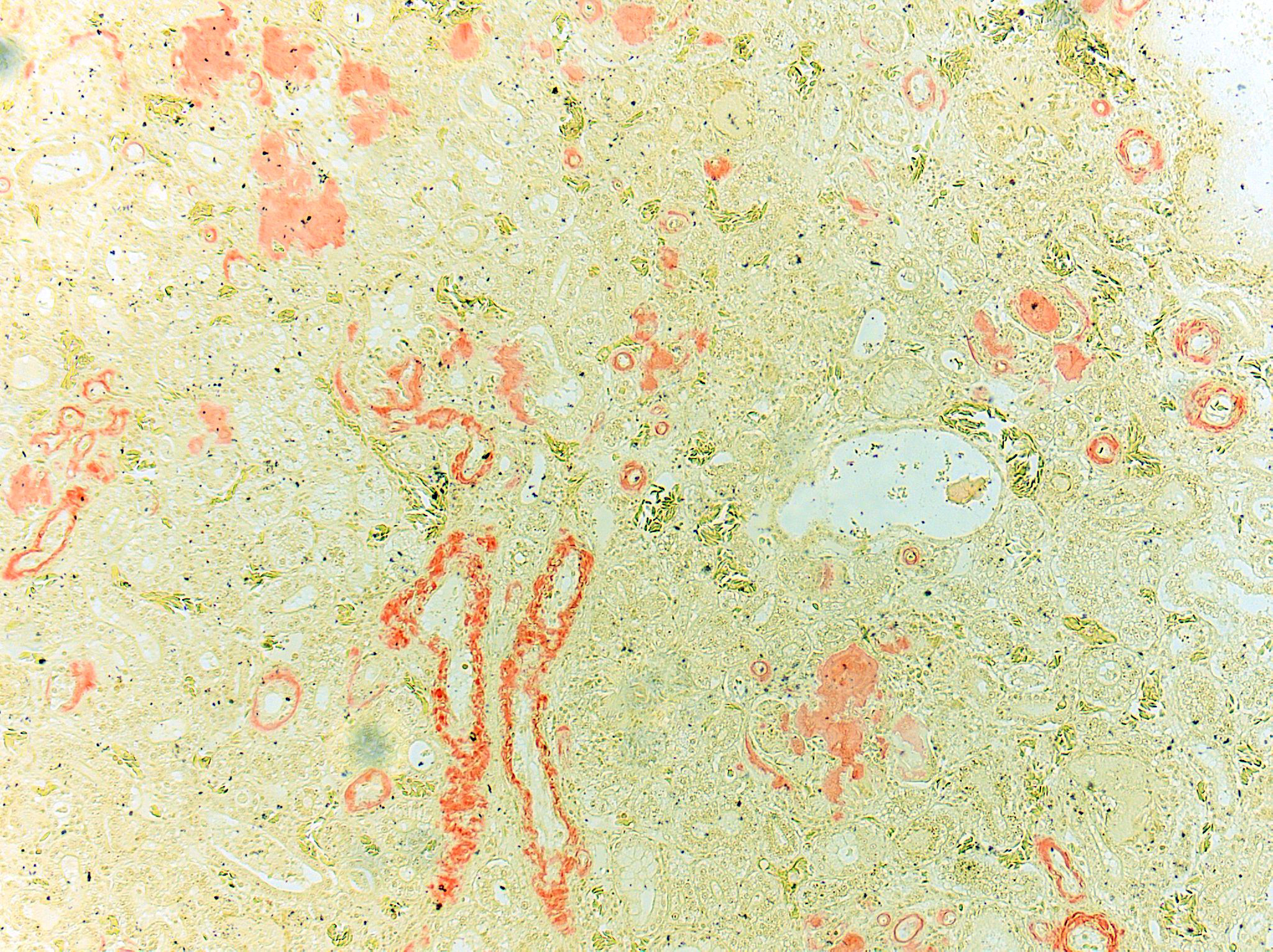
Congo Red and Von Kossa histochemical stains: Within glomeruli, tubular basement membranes, and walls of renal arteries and arterioles, there is congophilic pink-orange acellular material that exhibits strong apple-green birefringence when viewed under polarized light. Mineral deposits within renal tubules exhibit strong staining for calcium.
Contributor’s Morphologic Diagnosis:
- Kidney: Diffuse global glomerular amyloidosis with synechiae; moderate to marked renal tubular amyloidosis with degeneration, necrosis, loss, and regeneration with multifocal mineralization; and moderate, chronic, multifocal arteriolar amyloidosis.
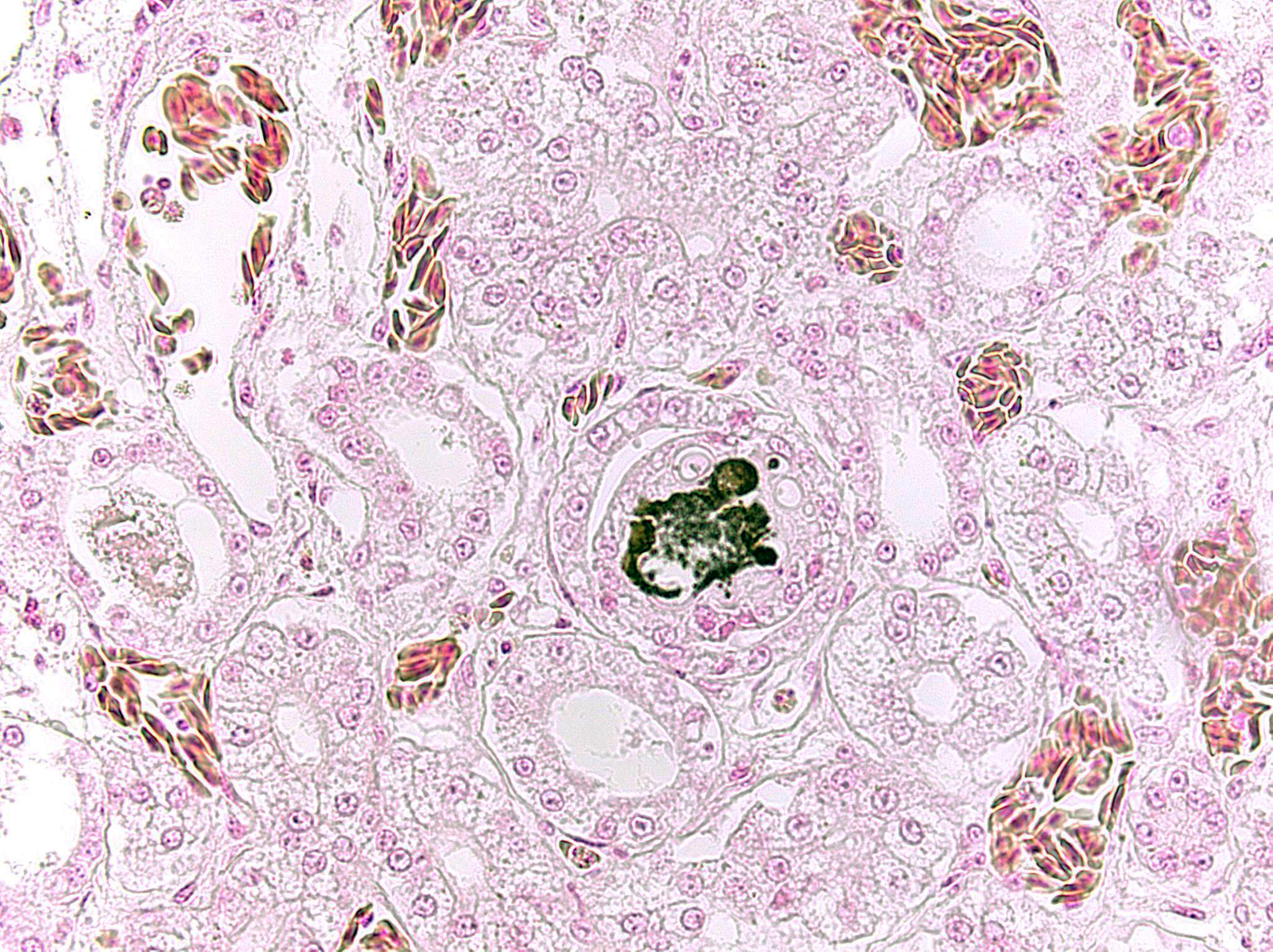
- Kidney: Multifocal urate tophi (presumed) with marked, chronic, multifocal histiocytic and lymphoplasmacytic interstitial nephritis.
- Kidney: Moderate to marked multifocal tubular ectasia with cellular casts.
Contributor’s Comment:
This is a case of confirmed systemic amyloidosis and visceral gout in a flamingo. Amyloidosis in this case is presumably secondary to chronic pododermatitis, as this association has been well-documented in flamingos with this condition. Amyloidosis, pododermatitis, and gout are all reviewed below.
Amyloidosis: Systemic AA (reactive) amyloidosis is an important cause of death in domestic and captive wild birds. AA amyloid is derived from serum amyloid A (SAA), an acute phase lipoprotein produced by hepatocytes. Chronic inflammatory diseases or neoplastic conditions can lead to excessive synthesis of SAA and protein misfolding resulting in amyloidosis.2,8,11 Several cytokines can induce hepatic synthesis of SAA including IL-1, IL-6, and TNF-α. SAA typically has an immuno-modulatory role in the inhibition of fever caused by IL-1β and TNF-α as well as the inhibition of platelet aggregation, suppression of thromboxane synthesis, and serotonin release from platelets.8 While an increased pool of precursor protein is necessary for the development of amyloidosis, certain amino acid substitutions may favor amyloidogenicity and give rise to unstable intermediate proteins that can easily reshape into fibrils.8
Two types of amyloidosis have been identified in birds – AA amyloidosis and cerebral Aβ amyloidosis accompanied by cerebral amyloid angiopathy. Avian AA amyloidosis commonly affects waterfowl, small passerine birds, and chickens. Aβ amyloidosis has been reported in great spotted woodpeckers and eagles but not in flamingos.11
AA amyloid deposition occurs most frequently in the liver, spleen, kidneys, and small intestine with the proventriculus, large intestine, heart, gonads, and endocrine organs being less affected.8 While lung, skin, and brain are rarely affected, vascular AA amyloid deposition has been reported in the central and peripheral nervous systems of flamingos.11
Grossly, affected organs are swollen, pale, and have a waxy consistency. The presence of amyloid can occasionally be demonstrated at the time of necropsy by applying Lugol’s iodine to the cut surface and rinsing with sulfuric acid, rendering amyloid deposits dark brown or black. Amyloid accumulation results in organ dysfunction and may lead to organ rupture due to disruption of tissues by the abnormal protein. Affected tissues are typically friable, and hepatic fractures with subsequent exsanguination are a common cause of acute death.1
Histologically, amyloid is acellular, homogenous, eosinophilic material that commonly accumulates in hepatic sinusoids, renal glomeruli, perifollicular areas of the spleen, lamina propria of the intestines, and vascular walls.1 The histologic diagnosis must be confirmed with Congo Red staining where amyloid stains peach or pink-orange and exhibits green birefringence under polarized light. AA amyloid can be confirmed via elimination of Congo Red staining affinity by oxidation of tissue sections in potassium permanganate. After staining with thioflavine-T, amyloid exhibits bright yellow fluorescence.2 Amino acid sequencing and immunohistochemical techniques utilizing monoclonal and polyclonal anti-AA antibodies directed against amyloid fibril proteins can aid in the identification of amyloid.15 On electron microscopy, amyloid is characterized by nonbranching 7-10 nm-diameter fibrils. 2
Experimentally, avian amyloidosis can be induced by repeated inflammatory stimuli, such as inoculation with bacterial extracts or vaccination with oil-emulsified bacterins. Thus, antigenic stimulation with bacteria is considered to promote the development of avian AA amyloidosis. In addition, recent studies have suggested that avian amyloidosis is transmissible. Horizontal transmission of amyloidosis via ingestion of amyloid-contaminated feed or feces is suspected to occur among avian species.10
Chronic inflammation associated with pododermatitis has been linked to systemic amyloidosis.10 In this case, systemic amyloidosis is attributed to the patient’s history of bilateral pododermatitis. Amyloid deposition was confirmed via Congo Red staining in the kidney, spleen, and pancreas. Arterial mineralization was confirmed in the spleen via Von Kossa staining. Despite the multifocal medial mineralization noted in small blood vessels, there was no evidence of atherosclerosis in the great vessels. It is possible that some of the gross lesions noted in the heart and GI tract at the time of postmortem exam represent foci of mineralization and/or amyloidosis. The grossly described white, chalky material in the stifle may be consistent with articular gout. However, no joint tissue was provided for confirmatory histopathological examination.
Pododermatitis: Pododermatitis (also known as bumblefoot) has only been documented in captive birds, and influencing factors include flooring in water ponds, prolonged periods indoors, environmental temperatures, age, weight, and potentially dietary zinc availability.12,13,14 Foot lesions are classified as inflammatory or proliferative with inflammatory lesions being more common in younger birds and proliferative lesions more common in older birds.1 Gross lesions include individual to coalescing nodules of thickened and hyperkeratotic epidermis with fissures, papillary growths, erosions or ulcers overlying granulation tissue and inflammation. Early lesions include heterophilic dermatitis, hydropic epithelial degeneration, hyperplasia, serocellular crust formation, and pustules. Chronic lesions feature neovascularization, fibrosis, and infiltration by lymphocytes and macrophages.
Secondary bacterial infections with organisms, such as Fusophorum spp. and Staphylococcus aureus are common and can exacerbate tissue damage and inflammation.4 Fungal and bacterial agents have not been consistently found and no virus has been isolated in pododermatitis lesions.12 Poxviral infection is the major differential for pododermatitis lesions.
Gout: Gout occurs in birds, reptiles, great apes, and humans due to the lack of the enzyme uricase, which oxidizes uric acid to allantoin. Uric acid in these species is normally eliminated through a combination of glomerular filtration, reabsorption, and secretion. Urate deposition (gout) usually occurs due to impaired excretion by the kidneys or overproduction of uric acid leading to hyperuricemia and precipitation of monosodium urate crystals (tophi) on visceral and articular surfaces. Comparatively, Dalmatian dogs also cannot oxidize uric acid to allantoin due to a defect in uricase, predisposing them to urate urolithiasis. This defect is an inherited autosomal recessive trait and linked to a mutation in SLC2A9, which encodes for a glucose transporter.2,7
Gout in birds occurs in acute (visceral) and chronic (articular) forms, which differ in frequency, age of onset, sex predilection, gross and microscopic lesions, pathogenesis and causes.3 Lesions may appear grossly as white foci, streaks, or sheets on serosal surfaces and viscera. Articular forms of gout include chalky, white deposits in and around joints. Urate crystals are often leached out during tissue processing and may be detected as remnant, wispy, basophilic, “ghost” crystals. Thus, tissues fixed in alcohol rather than formalin may improve detection. Histologically, fine, radiating acicular clefts may be present in the absence of inflammation or associated with histiocytic, granulomatous, or mixed inflammation. Gout should be distinguished from pseudogout, which is composed of non-urate, calcium pyrophosphate crystals.4
In birds, acute (visceral) urate deposition is more common than chronic (articular) urate deposition. Acute urate deposition may occur at any age with renal lesions involving white, chalky precipitates. Visceral organs including liver, myocardium, spleen, and serosal surfaces are commonly affected, whereas soft tissues around the joints may or may not be involved. Microscopically, there is an inflammatory reaction around tophi in the kidney and viscera with usually no inflammation in the synovium. Acute urate deposition is typically due to renal failure with causes including dehydration, nephrotoxicity, nephrotropic infectious agents, vitamin A deficiency, urolithiasis, neoplasia, or immune-mediated glomerulonephritis.3 Avian astroviruses have been identified as causes of visceral gout in the chicken, duckling, and gosling.9
In contrast, chronic urate deposition typically occurs in mature birds with the kidney being unaffected. Soft tissues other than synovium are rarely involved. Joints of the legs, wing, spine, and mandible may be affected. Microscopically, granulomatous inflammation is present in the synovium. Chronic urate deposition is likely due to a metabolic defect in the secretion of urates by renal tubules. Causes include genetics and a high protein diet.3
In this case, it is difficult to determine the sequence of events. Presumably, the pododermatitis and chronic inflammation lead to systemic amyloidosis, which impaired renal function. Dehydration and/or impaired excretion of uric acid by the kidneys could have resulted in subsequent deposition of urates. The presumptive urate tophi in the kidney are correlated with the patient’s hyperuricemia and bilaterally enlarged kidneys and consistent with acute (visceral) gout.
Contributing Institution:
The Ohio State University
College of Veterinary Medicine
Department of Veterinary Biosciences
https://vet.osu.edu/biosciences
JPC Diagnosis:
- Kidney, glomeruli: Amyloidosis, segmental to global, diffuse, marked.
- Kidney, arteries, and tubular basement membranes: Amyloidosis, diffuse, mar-ked.
- Kidney, tubules: Urate tophi, multiple.
- Kidney: Nephritis, interstitial, chronic and lymphocytic, diffuse, mild to moderate.
JPC Comment:
Flamingos and amyloid are a classic combination, and the blessing continue in this case with the addition of visceral gout and a history of pododermatitis. The contributor does an admirable job of summarizing these three components of the flamingo party pack.
Pododermatitis, the presumed antigenic stimulus for the systemic amyloidosis in this case, has been reported in many birds, including raptors, water fowl, penguins, cocaktiels and, of course, flamingos.12 Varying degrees of plantar lesions were found in up to 100% of captive flamingos in one study, and these lesions were the reason for euthanasia or a secondary finding in 95% of flamingos in another.12 In contrast to classic bumblefoot lesions, which consist of nodules with central ulceration, flamingo pododermatitis often includes varying degrees of hyperkeratosis, fissues of varying depth, nodular lesions with or without ulceration, and papillomatous growths.12
A 2015 study attempted to characterized the pododermatitis lesions in a group of 19 flamingos from a zoological collection. Viral PCR assays for papillomaviruses and herpesviruses were performed and were all negative for papillomaviruses; unknown herpesviral DNA was detected in samples from three birds, but the inconsistent presence of the virus made this unlikely to be the sole etiologic agent.
An intriguing result of this study was the identification of Micrococcus-like bacteria invading the stratum corneum of juvenile flamingos. The bacteria had zoospores and segmented, branching pseudohyphae that were PAS positive and Gram negative.12 In addition to the invading bacteria, the epidermis was characterized by heterophilic exocytosis, serocellular crust formation, and hydropic degeneration of the keratinocytes, particularly within the stratum granulosum.12 The subjacent dermis was characterized by accumulations of heterophis and lymphocytes, increased vascular profiles, and increased intercellular matrix. Invasion of Micrococcus-like bacteria was present in all examined juvenile flamingos (except two severely autolyzed carcasses), but the bacteria were rare in biopsies taken from adult animals.12
The bacteria found in the diseased plantar skin was isolated, subjected to 16S rRNA sequencing, and found to be a novel species within the genus Arsenicicoccus, which was subsequently named A. dermatophilus.5 The Arsenicoccus genus comprises gram-positive, facultatively anaerobic, catalase-posivie, non-spore forming bacteria that have been isolated both from the environment and from animal sources.6
In the 2015 study, histologic lesions were detected in both non-lesional and lesional skin, and the authors suggest that the proliferative lesions of flamingo pododermatisis are a reaction to the inflammation caused by A. dermatophius, which is given a leg up by a skin barrier weaked by nutritional deficiencies and inappropriate husbandry practices.12
Conference participants were tickled pink with this visual feast of a slide. Leading discussion was this week’s moderator, Dr. Neel Aziz, Supervisory Veterinary Medical Director and Head of the Diagnostic Pathology Unit at the Smithsonian National Zoological Park and Conservation Biology Institute. Dr. Aziz began by noting that diseases seen in zoological collections differ in kind and severity than thos seen in the wild, as this case illustrates. As noted by the contributor, pododermatisis is a disease of animal under human care; it is not seen in the wild. And this individual animal, at 28 years old, is an aged animal that has had time to collect more pathology than a typical wild flamingo.
Some conference participants were initially confused by the appearance of the amyloid on this slide which had a more basophilic hue than typica amyloid. Dr. Aziz noted that this amyloid can take on a variety of appearance, appearing more typically eosinophilic or more amphophilic, even within the same animal.
Dr. Aziz discussed the typical appearance of gout, which is characterized by the deposition of needle-like monosodium urate crystals that typically do not survive processing and leave behind only negative, ghost tophi shapes in histologic sections. By contrast, the gout appreciated in this case is consisted of amphophilic, amorphous material which the moderator speculated might represent an early stage lesion. Dr. Aziz noted that the terminology of “visceral” and “articular” should be reserved for the reptiles; in birds, “acute” versus “chronic” is the preferred terminology, with acute urate deposition occurring in the viscera of birds and chronic urate deposition occurring in the joints.
In an embarrassment of riches, Dr. Karen Terio, Clinical Professor in the Zoological Pathology Program at the University of Illinois, was also in attendance at conference this week. Dr. Terio noted that it can be overwhelming to describe a slide with this complexity. She recommended organizing such descriptions by pathogenesis and would start a description of this slide with glomerular changes, as the observed tubular changes are likely secondary to the glomerular amyloidosis.
Conference discussion produced a flock of morphologic diagnoses largely in agreement with the contributor. Participants discussed the interstitial nephritis at length and whether it was only in response to the urate deposition or if the inflammation also appeared in areas of the interstitium untouched by tophi. Participants thought it likely that the interstitial nephritis could not be completely explained by the urate deposition and thus preferred to describe it in a separate morphologic diagnosis.
References:
- Buckles EL. Phoenicopteriformes. In: Terio KA, McAloose D, St. Leger J, eds. Pathology of Zoo and Wildlife Animals. Cambridge, MA, Academic Press; 2018: 687-688.
- Cianciolo RE, Mohr FC. Urinary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Vol 2. Elsevier; 2016:413-415,457.
- Crespo R, França MS, Fenton H, Shivaprasad HL. Galliformes and Columbiformes. In: Terio KA, McAloose D, St. Leger J, eds. Pathology of Zoo and Wildlife Animals. Academic Press; 2018:743-744.
- Fenton H, McManamon R, Howerth EW. Anseriformes, Ciconiiformes, Charadriiformes, and Gruiformes. In: Terio KA, McAloose D, St. Leger J, eds. Pathology of Zoo and Wildlife Animals. Academic Press; 2018:695-696,601-702.
- Gobeli S, Thomann A, Wyss F, Kuehni-Boghenbor K, Brodard I, Perreten V. Arsenicicoccus dermatophilus sp. nov., a hypha-forming bacterium isolated form the skin of greater flamingos (Phoenicopterus roseus) with pododermatitis. Int J Syst Evol Microbiol. 2013;63(11):4046-4051.
- Jeong JH, Kweon OJ, Kim HR, Kim TH, Ha SM, Lee MK. A novel species of genus Arsenicoccus isolated from human blood using whole-genome sequencing. Ann Lab Med. 2021;41(3):323-327.
- Karmi N, Brown EA, Hughes SS, et al. Estimated frequency of the canine hyperuricosia mutation in different dog breeds. J Vet Intern Med. 2010; 24(6):1337-1342.
- Landman WJM, Gruys E, Gielkens ALJ. Avian amyloidosis. Avian Pathol. 1998; 27(5): 437-449.
- Li L, Sun M, Zhang Y, Liao M. A review of the emerging poultry visceral gout disease linked to avian astrovirus infection. Int J Mol Sci. 2022; 23, 10429.
- Murakami T, Ishiguro N, Higuchi K. Transmission of systemic AA amyloidosis in animals. Vet Pathol. 2014;51(2):363-371.
- Ono A, Nakayama Y, Inoue M, Yanai T, Murakami T. AA amyloid deposition in the central and peripheral nervous systems in flamingos. Vet Pathol. 2020;57(5):700-705.
- Wyss F, Schumacher V, Wenker C, et al. Pododermatitis in captive and free-ranging greater flamingos (Phoenicopterus ros-eus). Vet Pathol. 2015;52(6):1235-1242.
- Wyss F, Wenker C, Hoby S, et al. Factors influencing the onset and progression of pododermatitis in captive flamingos (Phoenicopteridae). Schweiz Arch Tierheilkd. 2013;155:497-503.
- Wyss F, Wolf P, Wenker C, et al. Comparison of plasma vitamin A and E, copper and zinc levels in free-ranging and captive greater flamingos (Phoenicopterus ros-eus) and their relation to pododermatitis. J Anim Physiol and Anim Nutr. (Berl) 2014; 98:1102-1109.
- Zschiesche W, Linke RP. Immunohistochemical characterization of spontaneous amyloidosis in captive birds as AA-type, using monoclonal and polyclonal anti-AA antibodies against mammalian amyloid. Acta Histochem. 1989;86:45-50.