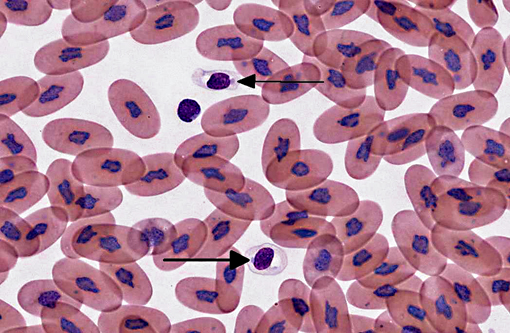
Signalment:
Gross Description:
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Complete Blood Count:
| Parameter | Reference3 | |
| WBC (x103/μL) | 40 H | 4 10 |
| Heterophils (x103/μL) | 4 | 0.8 6.5 |
| Lymphocytes (x103/μL) | 34.8 H | 0.4 6 |
| Azurophils (x103/μL) | 1.2 H | 0 0.58 |
| Eosinophils (x103/μL) | 0 | 0 0.3 |
| Basophils (x103/μL) | 0 | 0 2 |
| Thrombocytes | Adequate | N/A |
| PCV (%) | 37 | 24 40 |
| Total Protein (g/dL) | 9.7 H | 4.6 8.0 |
Condition:
Contributor Comment:
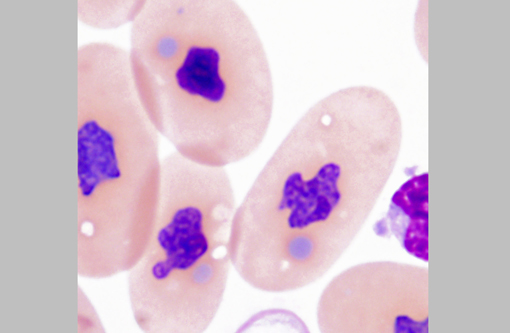
The diagnosis of IBD relies on identifying the characteristic intracytoplasmic inclusions. These inclusions can be found antemortem in blood films or buffy coat preparations.(3,5,8,11) In blood films, inclusions can be found in lymphocytes, erythrocytes, or heterophils.(3) IBD inclusions stained with Wright-Giemsa are intracytoplasmic and lightly basophilic with variable size and shape.(3,8) The diagnosis of IBD can be confirmed with H&E staining, in which case, these inclusions stain eosinophilic to amphophilic.(3,5) However, not all histologically confirmed IBD cases have easily identifiable inclusions in the circulating blood.(5) On postmortem examination, the inclusions can be found in glial cells and neurons of the central nervous system, lymphocytes in lymphoid organs, and epithelial cells of internal organs (gastrointestinal tract, liver, pancreas, etc).(3,7,11) In the current case, histopathology revealed inclusions in lymphocytes in the spleen, in hepatocytes, and pancreatic epithelium.
There are several reports of C-type retrovirus-like budding particles in IBD inclusions with TEM.(6,7,9,11,12) However, a recent report suggests that the inclusions are instead composed of aggregates of a unique, non-viral 68 kD IBD protein.(3) To the authors knowledge, there are no TEM descriptions of inclusions in circulating lymphocytes. The inclusions described here consisted of large aggregates of non-membrane bound amorphous granular electron dense material without viral-like particles.Â
When snakes demonstrating clinical signs are diagnosed with IBD the prognosis is grave, as the disease is always fatal.(11) However, the long term survival of asymptomatic animals with IBD has not been evaluated. This case highlights the importance of evaluating blood films of susceptible snakes for IBD inclusions. Although there may be poor sensitivity, a blood film or buffy coat evaluation is a cheap, non-invasive test that can be used to detect this disease, especially if there is a lymphocytosis.Â
**Note: Portions of this case have been published Banajee KH, Chang L, Jacobson ER, Rich GA, Royal AB. Whats your diagnosis? blood film from a boa constrictor. Vet Clin Path. 2012;41(1):158-159.
JPC Diagnosis:
Conference Comment:
Additionally, participants discussed characteristics of reptilian blood cells and reptilian hemoparasites that can be found on blood films. In reptiles, mature erythrocytes are larger than their mammalian or avian counterparts, and, as in birds, reptile erythrocytes are ellipsoidal with centrally-placed, oval to round nuclei with dense purple chromatin and irregular margins.2 Often, small (0.5 μm to 2.0 μm) round to irregular, basophilic intracytoplasmic inclusions are found in the cytoplasm of reptile erythrocytes and are suspected to be degenerate organelles, likely due to slide preparation artifact. Likewise, the basophilic stippling associated with a regenerative response or lead poisoning should not be confused with pathogen associated intracytoplasmic inclusions. Reptilian lymphocytes are similar in appearance and function to mammalian and avian lymphocytes. Circulating monocytes (azurophils) are usually found in small numbers; however, increased numbers are seen with granulomatous inflammation. Granulocytes in reptiles are classified as acidophils (heterophils and eosinophils) and basophils. Heterophils have bright orange fusiform cytoplasmic granule, and function similarly to avian heterophils (i.e. they rely heavily on oxygen-independent mechanisms to destroy phagocytized microbes.) Eosinophils contain eosinophilic spherical granules, and function much like their mammalian counterparts. Basophils appear and function similarly to those found in mammals and birds; however, their relative number can be higher and in some species comprise up to 40% of the leukocyte differential. Reptilian thrombocytes are elliptical to fusiform with a centrally-positioned nucleus, dense chromatin and colorless cytoplasm with few azurophilic granules.(2)
Hemoparasites and microfilaria are common in reptiles and are often encountered on blood films. Although usually considered incidental, some can cause disease (i.e. hemolytic anemia).(2) Common hemoprotozoa in reptiles include hemogregarines (Hemogregarina, Hepatozoon, Karyolysus), trypanosomes and Plasmodium spp. Less common hemoprotozoans of reptiles include Leishmania, Saurocytozoon, Haemoproteus, Schellackia, and the piroplasmids. Hemogregarines are identified in erythrocytes by the presence of intracytoplasmic gametocytes that lack refractile pigment granules. Trypanosomes are large extracellular flagellate protozoa. Plasmodium trophozoites are small, signet-ring structures found in erythrocyte cytoplasm and have gametocytes with refractile granules; whereas Sauroleishmania appear as round to oval 2-4 μm blue organisms with an oval, red nucleus in the cytoplasm of thrombocytes or mononuclear leukocytes. Saurocytozoon can be identified by their round gametocytes that lack pigment granules in the cytoplasm of lizard leukocytes. Haemoproteus gametocytes have refractile pigment granules and, similar to Haemoproteus in birds, can cause dehemoglobinization of the infected erythrocyte.(2) Lainsonia and Schellackia are coccidian parasites whose sporozoites appear as intracytoplsasmic inclusions in erythrocytes and lymphocytes of lizards and snakes. The sporozoites are round to oval, pale staining, nonpigmented inclusions that deform the host cell nucleus into a crescent shape. Piroplasmids such asBabesia, Aegyptianella, Sauroplamsa or Serpentoplasma appear as small nonpigmented, round to piriform and signet ring-like inclusions in erythrocyte cytoplasm.
References:
2. Campbell TW. Hematology of reptiles. In: Thrall MA, Weiser G, Allison RW, Campbell TW, eds. Veterinary Hematology and Clinical Chemistry. 2nd ed. Ames, Iowa: Wiley-Blackwell; 2012:Kindle edition, retrieved from Amazon.com; location 34%).
3. Chang L, Jacobson ER. Inclusion body disease, a worldwide infectious disease of boid snakes: a review.J Exotic Pet Med. 2010;19(3):216-225.
4. Diethelm G, Stein G. Hematologic and blood chemistry values in reptiles. In: Mader DR, ed. Reptile Medicine and Surgery. 2nd ed. St. Louis, MO: Saunders Elsevier; 2006:1103-1118.
5. Garner MM. Methods for diagnosing inclusion body disease in snakes. Proc North Am Vet Conf. 2005;19:1283-1284.Â
6. Jacobson ER, Oros J, Tucker S, et al. Partial characterization of retroviruses from boid snakes with inclusion body disease. Am J Vet Res. 2001;62(2):217-224.
7. Or³s J, Tucker S, Jacobson ER. Inclusion body disease in two captive boas in the Canary Islands. Vet Rec. 1998;143(10):283-285.
8. Pees M, Schmidt V, Marschang RE, Heckers KO, Krautwald-Junghanns M-E. Prevalence of viral infections in captive collections of boid snakes in Germany. Vet Rec.Â
2010;166(14):422-425.Â
9. Schumacher J, Jacobson ER, Homer BL, Gaskin JM. Inclusion body disease in boid snakes. J Zoo Wildl Med. 1994;25(4):511-524.Â
10. Stenglein MD, Sanders C, Kistler AL, et al. Identification, characterization, and In Vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: candidate etiological agents for snake inclusion body disease. mBIO. 2012;3(4):1-12.
11. Vancraeynest D, Pasmans F, Martel A, et al. Inclusion body disease in snakes: a review and description of three cases in boa constrictors in Belgium. Vet Rec. 2006;158(22): 757-761
12. Wozniak E, McBride J, DeNardo D, Tarara R, Wong V, Osburn B. Isolation and characterization of an antigenically distinct 68-kd protein from nonviral intracytoplasmic inclusions in boa constrictors chronically infected with the inclusion body disease virus (IBDV: Retroviridae). Vet Path. 2000;37(5):449-459.