CASE 2: 17-H43332 (4100929-00)
Signalment: 4-year-old, male, castrated, Yorkshire terrier dog (Canis familiaris)
History:
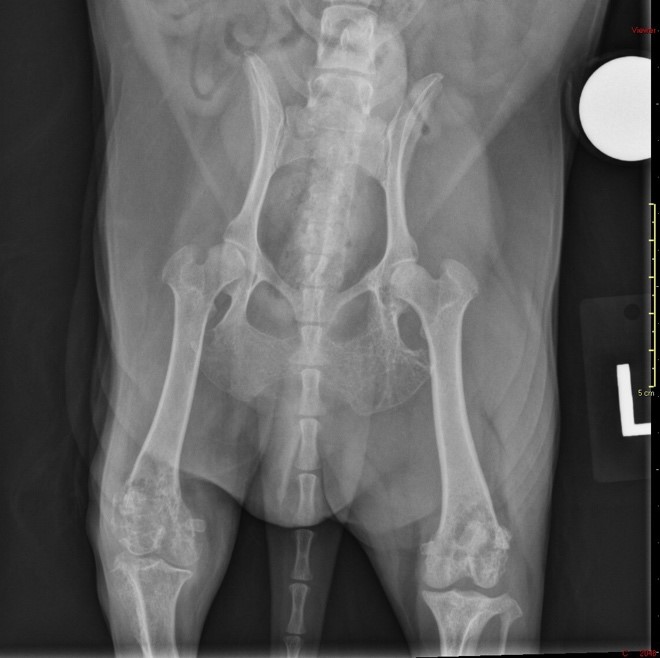
This dog presented for evaluation of chronic (1 year) right hind limb lameness that had recently become significantly worse ? the dog was non-weight bearing on the right hindlimb. Additionally, the dog became lame on the left hind limb and was having significant trouble walking. Radiographs revealed moth-eaten lucencies of the left ischium, left acetabulum, left and right distal femurs, fabellae, right proximal tibia, right patella, caudal aspect of both scapulae, and a suspected pathologic fracture of the right tibial tuberosity. A Blastomyces urine antigen test was negative. Bloodwork revealed a hyperglobulinemia (total protein: 9.8 g/dL) and a fine needle aspirate of bone was inconclusive. The dog was originally from Arizona and had since moved to Illinois approximately four years ago.
Gross Pathology:
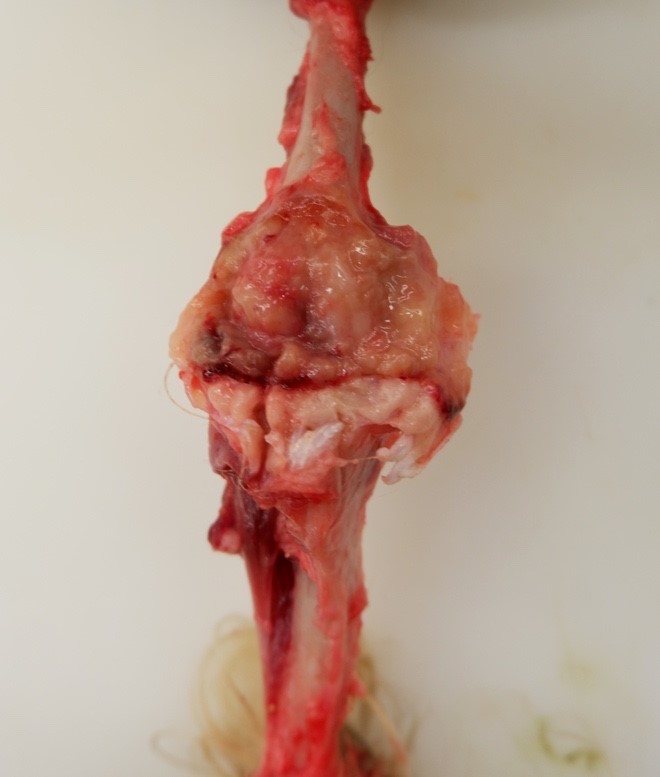
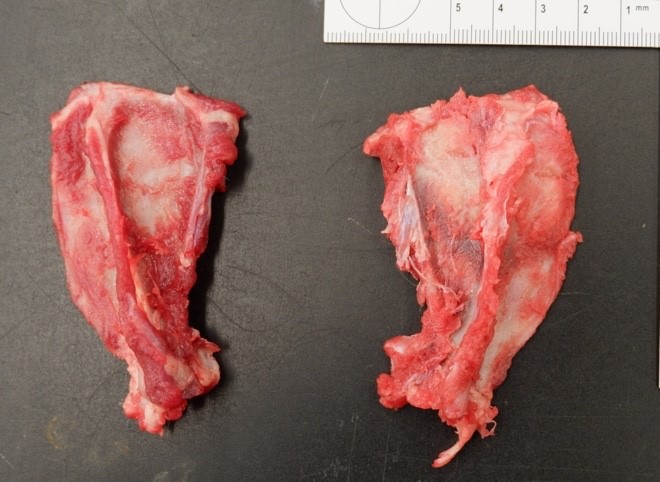
Musculature of the right hind limb was generally and moderately reduced compared to the left hind limb (atrophy). The right stifle joint was swollen with crepitus palpated upon extension, contains a moderate amount of thick, tan, opaque material, and the joint capsule is mildly thickened, tan, and granular. The distal portion of the right lateral femoral condyle was freely moveable and jagged (pathologic fracture). The trochlear ridges and condyles of the right and left femur were irregularly raised, roughened, translucent (thinned), and easily cut by a scalpel blade. The left and right ischiatic tuberosities were asymmetrical with the surface being slightly expanded, roughened, irregular, thinned, and easily cut by a scalpel blade. The dorsal half of the right scapula was also mildly expanded, roughened, thinned, and easily cut by a scalpel blade.
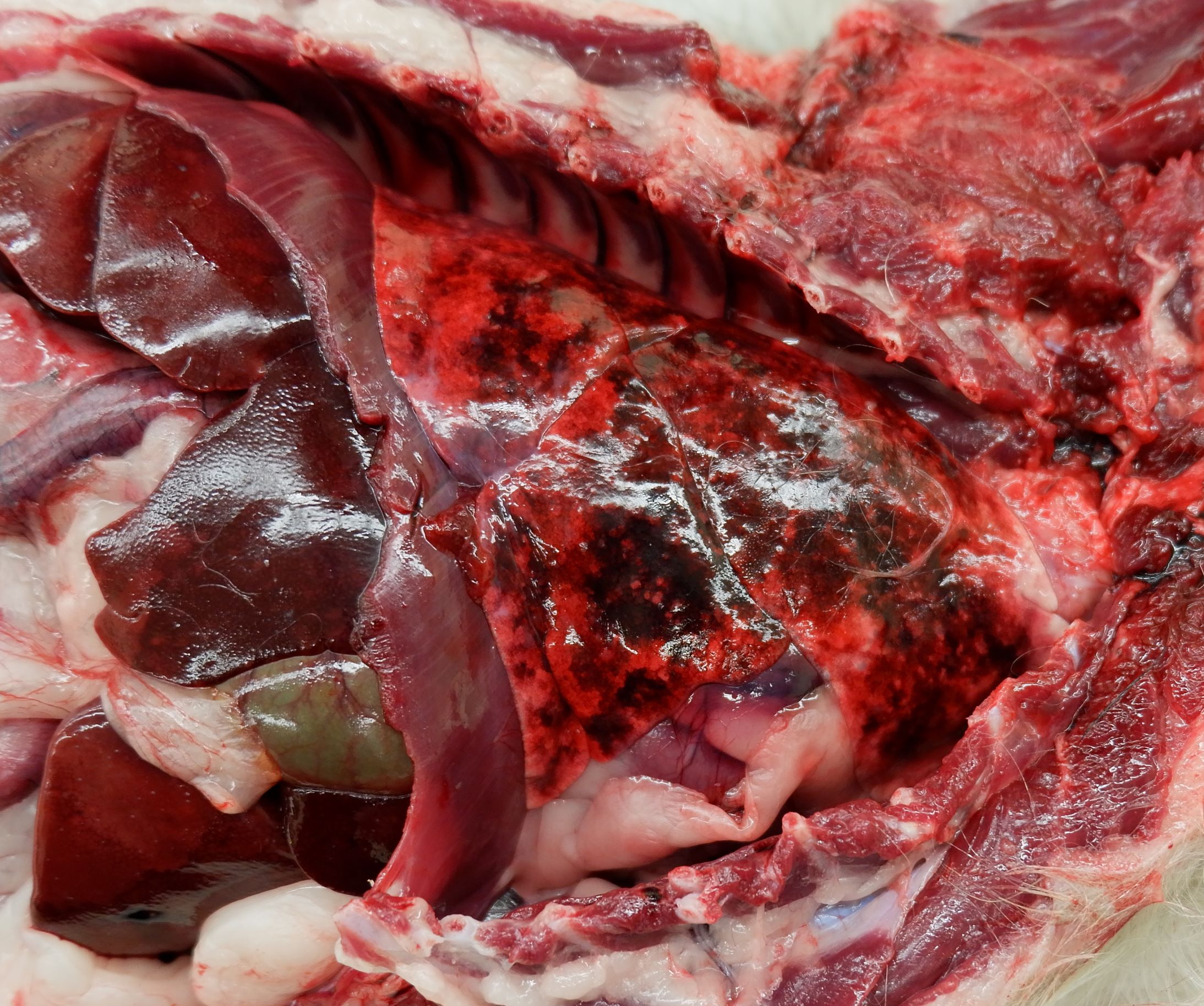
The right popliteal and bilateral inguinal lymph nodes were markedly enlarged, firm, white, and granular on cut surface.
The lungs were diffusely mottled red to pink, soft, and contained multiple, randomly scattered, 1-3 mm diameter, white, semi-firm, nodules within all lung lobes. The pleural surface of the diaphragm, at the junction of the central tendon and muscle, contained several similar nodules.
The nasal cavity and brain were unremarkable.
Laboratory results:
A portion of joint capsule was submitted for aerobic and myocology culture which returned a light growth of Coccidioides sp. and very rare growth of Pasteurella stomatis.
The Coccidioides sp. isolate from mycology culture was identified by 18S rRNA gene sequencing with the top match being similar to C. posadasii (100%) but also with a very close homology to C. immitis (99%).
Microscopic description:
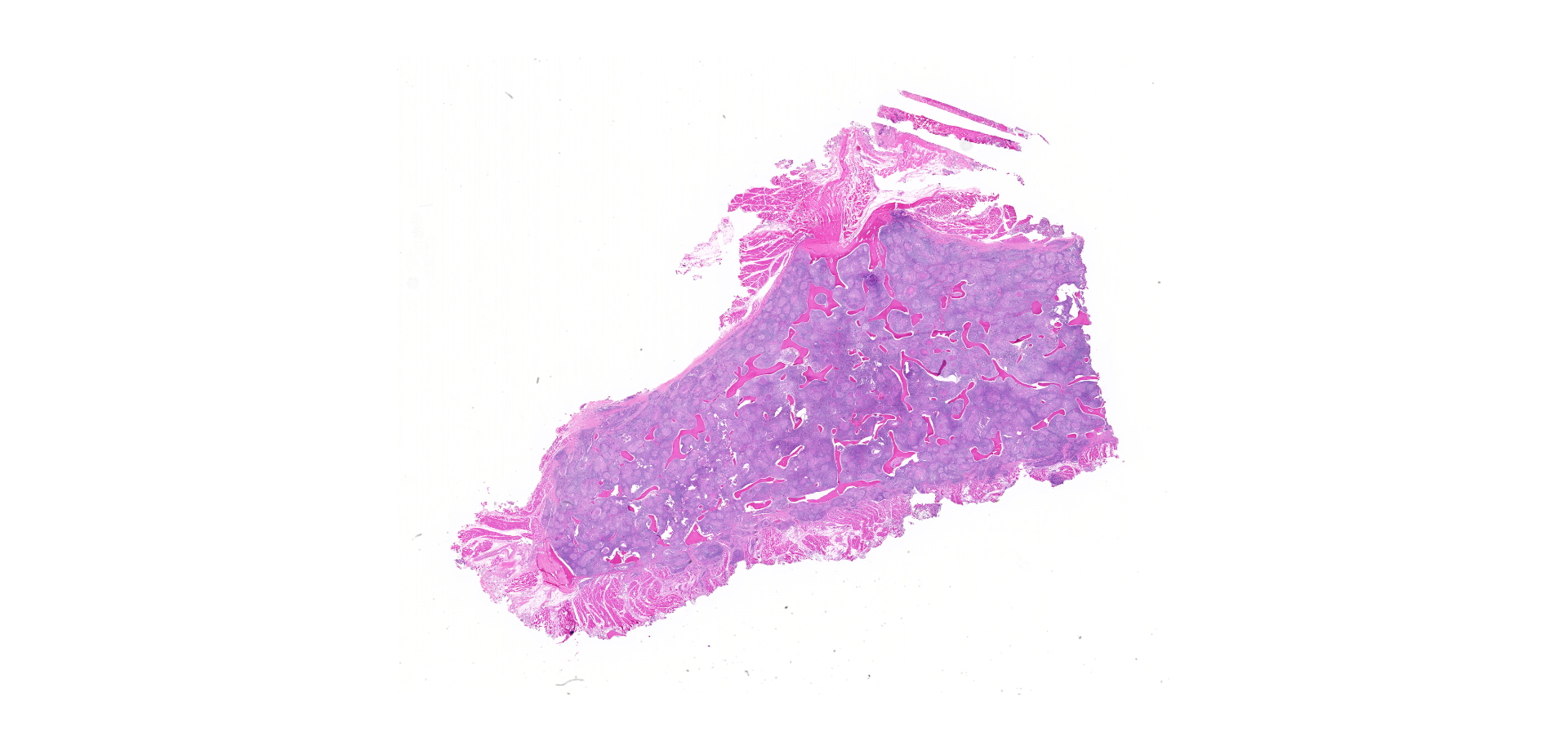
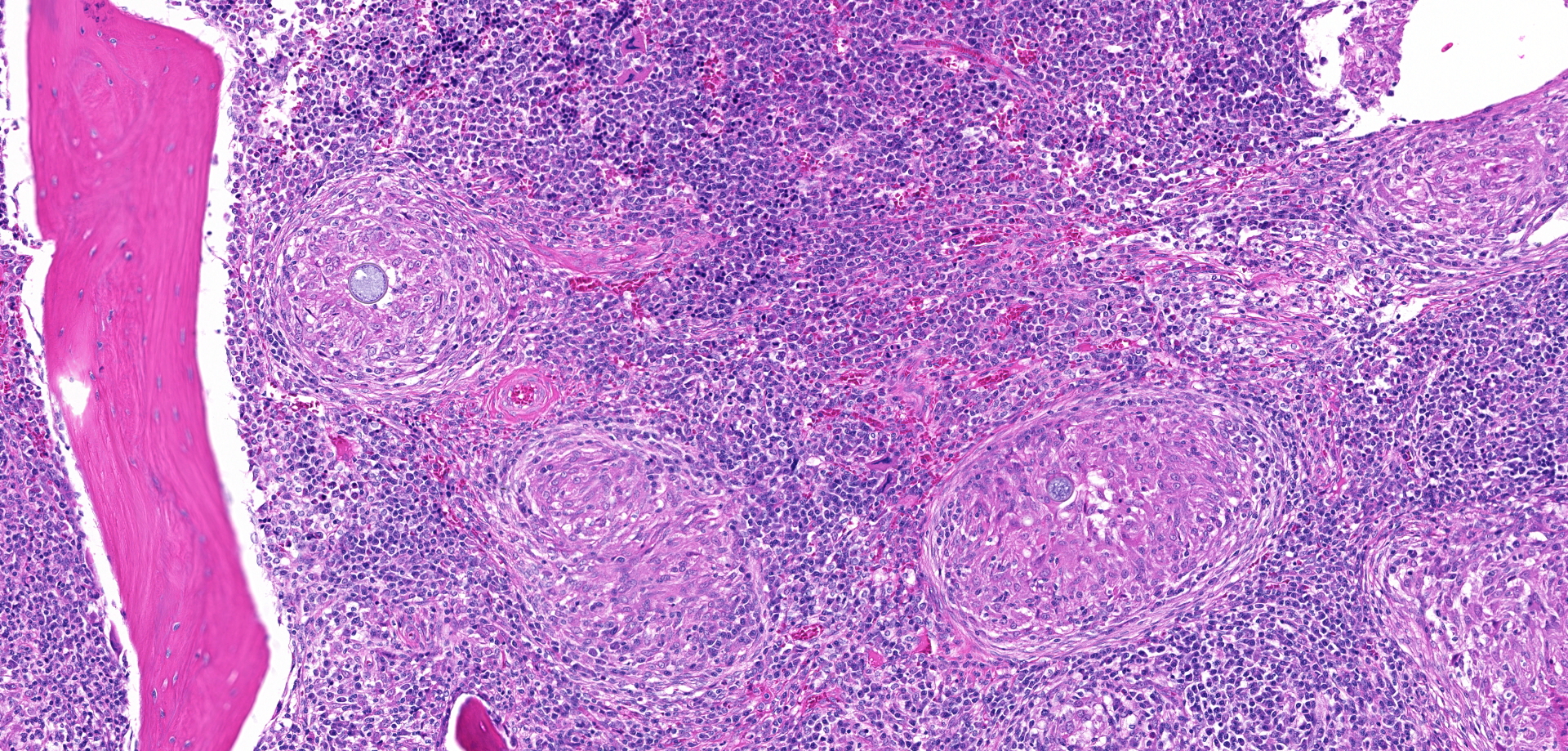
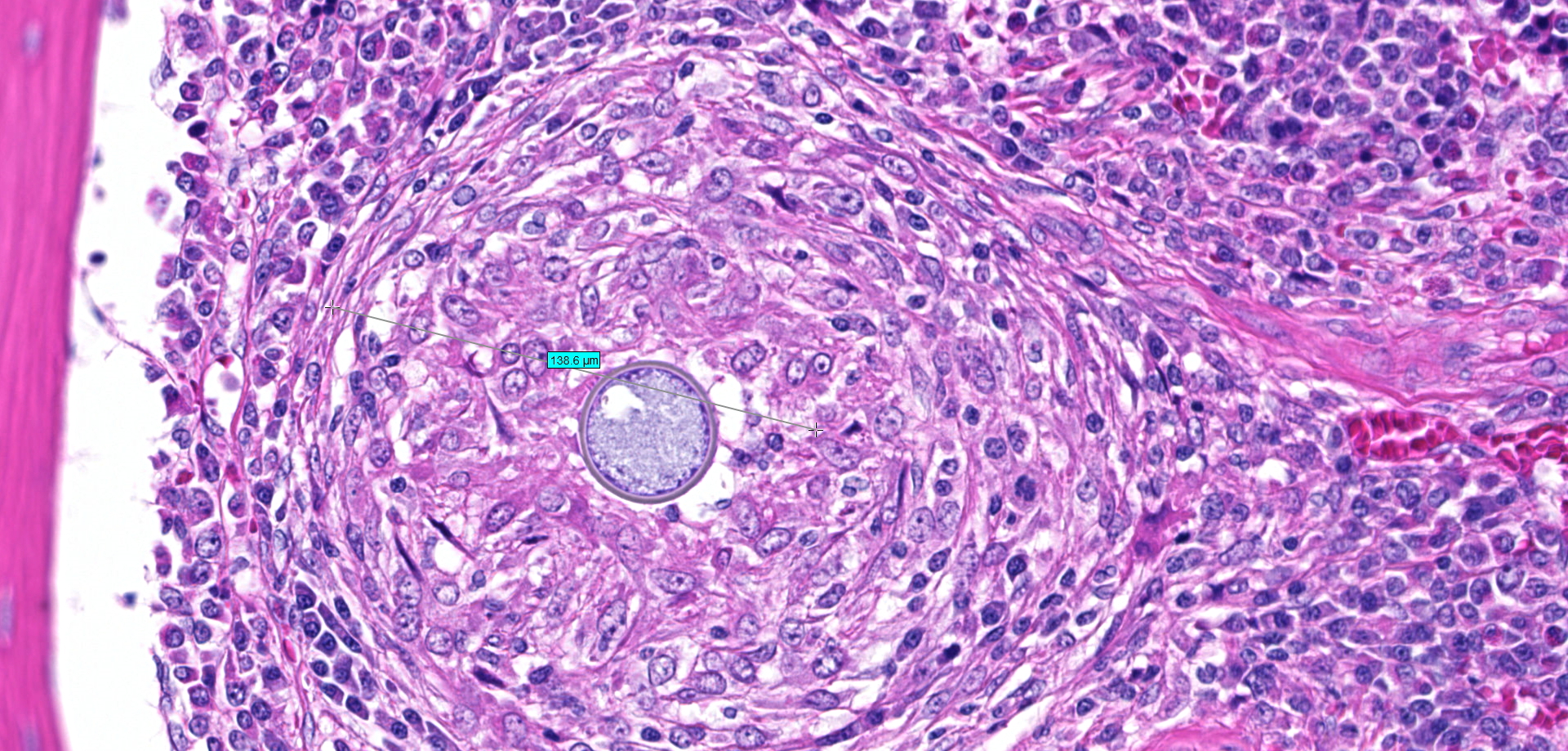
Bone (right femur and scapula): The medullary space is effaced and expanded by numerous coalescing nodules of mixed inflammation suspended in thin anastomosing bands of dense fibrous connective tissue. The inflammatory nodules comprise mostly epithelioid macrophages and rare neutrophils surrounded by a layer of lymphocytes and plasma cells and occasionally encapsulated by a thin band of fibrous connective tissue (granulomas). The center of granulomas is occasionally replaced by granular to amorphous (necrotic) debris and often contain at least one discrete fungal spherule. The spherules are 20-60 um in diameter characterized by a smooth, 5 um thick, refractile and hyaline, double-contoured wall and contain granular basophilic material or rarely a few 5-7 um diameter endospores. The trabecular and cortical bone are markedly thinned and often obliterated by the inflammation. The thin trabeculae of remaining bone are usually smooth, occasionally scalloped, and rarely lined by small numbers of osteoclasts located within Howship's lacunae. Within sections of the distal femur, the same inflammatory response obliterates the articular cartilage, extends into the joint space, and expands and effaces the synovium of the stifle joint. In the section of scapula, the inflammation multifocally obliterates the cortical bone and extends into the adjacent skeletal muscle.
Contributor's morphologic diagnosis:
Bone (right femur and scapula): Severe, chronic, multifocal, granulomatous osteomyelitis, arthritis, and synovitis, with extensive boney lysis and intralesional fungal spherules (consistent with Coccidioides sp).
Contributor's comment:
Coccidioides is a soil dwelling, dimorphic fungus that can cause systemic disease in a wide range of mammals, humans, and some reptiles.13 Coccidioidomycosis is also known as San Joaquin Valley fever, valley fever, or desert fever and is endemic to semiarid regions of southern Arizona, California, New Mexico, Nevada, Utah, western Texas, northern Mexico, and Central and South America.6,11,12 Distinction between two different phylogenetic clades based on genetic analysis suggests that there are two different species that cause disease including Coccidioides immitis and Coccidioides posadasii, however the biological relevance of this genetic variability is unclear.3,5
During periods of heavy rainfall, the dormant mycelium below the soil surface proliferates and extends to the surface where it produces infective spores (arthroconidia). When disturbed by animal activity, these infective arthroconidia are inhaled or, less commonly, come into contact with damaged skin, where only a few spores are required to initiate infection. After being inhaled, the arthrospores are phagocytosed in alveoli, which stimulates structural changes in the arthrospores to form into spherules. These spherules swell, undergo endosporulation, rupture, and release hundreds of endospores into the surrounding tissue. The newly released endospores mature into new spherules and the cycle is repeated within affected tissues.3,6,12
Because of the fungal life cycle, dogs in endemic areas that spend more time outdoors, have more land to roam, and exposed to dusty environments are at increased risk of infection. Other risk factors in dogs include a younger age, being a large breed, and working or sporting activity.1,15 Clinical infection is commonly localized to the pulmonary system and associated lymph nodes resulting in chronic respiratory disease. However, when endospores enter the lymphatics or blood, tropism for bones, joints, and less frequently heart, brain, eyes, testes, skin, spleen, liver, and kidney create a wide range of clinical signs that can complicate antemortem diagnosis.6 Dissemination can occur early in the disease process or months after initial infection, with the most common clinical presentations being neurological signs or lameness due to osteomyelitis. Diagnosis often relies on multiple modalities including clinical suspicion based on physical exam, signalment, and history, routine bloodwork, radiographs, serology, and cytology or histopathology. Serological tests (including agar gel immunodiffusion, ELISA, and latex particle agglutination) can be specific, however they are typically insensitive and can produce false negative results.6,8 Detection of antigen in urine or serum is also an insensitive test for diagnosis in dogs.10 Therefore, cytological examination of trans-tracheal wash, bronchoalveolar lavage fluid, and fine needle aspirates and/or histopathological examination of affected tissues are often pursued to help provide a definitive diagnosis. Fungal culture is another diagnostic option; however, this procedure presents a hazard to laboratory personnel and should only be performed in properly equipped laboratories.6,8
Contributing Institution:
University of Illinois College of Veterinary Medicine, Veterinary Diagnostic Laboratory
http://vetmed.illinois.edu/vet-resources/veterinary-diagnostic-laboratory/
JPC diagnosis:
Bone: Osteomyelitis, granulomatous, diffuse, severe, with marked bone lysis and numerous endosporulating spherules, Yorkshire terrier, canine.
JPC comment:
The first documented case of coccidioidomycosis was in 1892 in an Argentinian soldier who presented with skin lesions. While it resembled mycosis fungoides, this soldier's lesions differed, and had rounded organisms at the center of lesions. Originally thought to be a protozoan, the name Coccidioides was given because of the similarities with coccidia. It wasn't until 8 years later that it was reclassified as a fungus, but the name remained the same.2
Coccidioides immitis and Coccidioides posadasii are indistinguishable via serology, they are morphologically identical, and their predicted proteins are >90% homologous. The largest factor for differentiating these two species is geography, with C. immitis found primarily in the desert regions of central and southern California, while C. posadasii is primarily found in desert regions of Nevada, Arizona, New Mexico, western Texas, Mexico, and Central and South America. There are significant genetic variations between the two species, allowing for differentiation.9
While there are gaps in knowledge regarding virulence factors, it has been shown that deletion of the gene coding for the spherule outer wall glycoprotein decreases virulence significantly. Ammonia metabolism is important for virulence as well, and Coccidioides spp synthesize a urease and ureidoglycolate hydrolase, allowing for the alkalization of the local environment. Additionally, the gene encoding carbamoyl phosphate synthetase, CPS1, appears to be significant to virulence, as deletion of the gene resulted in slow spherule growth, lack of endosporulation, and was avirulent in immunocompromised mice.9
The host response is multifactorial, but both innate and adaptive immune systems serve necessary functions of a successful response. Of the pattern recognition receptors (PRR), the most active in responding to the Coccidioides spp spherule wall are Toll-like receptors (TLR) and C-type lectin-like receptors (CLR). Specifically, the gene Clec7a encodes C-type receptor Dectin-1, a b-glucan receptor that responds to fungal cell walls. Downstream from Dectin-1 is caspase recruitment domain-containing protein 9 (Card9), which is an immune adaptor protein that activates Th1 and Th17 immunity against Coccidioides spp infection.7
The environment plays an important role in the ability of Coccidioides sp. to survive and proliferate. In areas with endemic Coccidioidomycosis, the soil has high levels of essential nutrients such as iron, calcium, and magnesium, regardless of soil pH. Animals carcasses may play an important role in the survival of this fungus as a medium for fungal growth, with isolates of Coccidioides sp. found around animal burrows, with no isolates found in areas with no animal activity.4
References:
1. Butkiewicz CD, Shubitz LF, Dial SM. Risk factors associated with Coccidioides infection in dogs. J Am Vet Med Assoc. 2005;226(11):1851-1854.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin N Am. 2003;17:41-57.
3. Cox RA, Magee DM. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev. 2004;17(4):804-839.
4. del Rocio Reyes-Montes M, Ameyali Perez-Huitron M, Luis Ocana-Monroy J, et al. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infectious Diseases. 2016;16:550.
5. Fisher MC, Koenig GL, White TJ, et al. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002;94(1):73-84.
6. Graupmann-Kuzma A, Valentine BA, Shubitz LF, et al. Coccidioidomycosis in dogs and cats: a review. J Am Vet Med Assoc. 2008;44(5):226-235.
7. Hung CY, Hsu AP, Holland SM, Fierer J. A review of innate and adaptive immunity to coccidioidomycosis. Medical Mycology. 2019;57:S85-S92.
8. Johnson LR, Herrgesell EJ, Davidson AP, et al. Clinical, clinicopathologic, and radiographic findings in dogs with coccidioidomycosis: 24 cases (1995?2000). J Am Vet Med Assoc. 2003;222(4):461-466.
9. Kirkland TN, Fierer J. Coccidiodes immitis and posadasii; A review of their biology, genomics, pathogenesis, and host immunity. Virulence. 2018;9(1):1426-1435.
10. Kirsch EJ, Greene RT, Prahl A, et al. Evaluation of Coccidioides antigen detection in dogs with coccidioidomycosis. Clin Vaccine Immunol. 2012;19(3):343-345.
11. Nguyen C, Barker BM, Hoover S, et al. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev. 2013;26(3):505-525.
12. Shubitz LF, Butkiewicz CD, Dial SM, et al. Incidence of Coccidioides infection among dogs residing in a region in which the organism is endemic. J Am Vet Med Assoc. 2005;226(11):1846-1850.
13. Shubitz LF. Comparative aspects of coccidioidomycosis in animals and humans. Ann N Y Acad Sci. 2007:1111:395-403.