Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2017-2018
Conference 25
May 9th, 2018
CASE III: CASE 2 51304 (JPC 4068392)
Signalment: 3-week-old, female, Jersey (Bos taurus), bovine.
History: The calf had a reported history of a rotavirus scour and was treated with antibiotics and supportive therapy that consisted of tube-feeding milk by the owner.
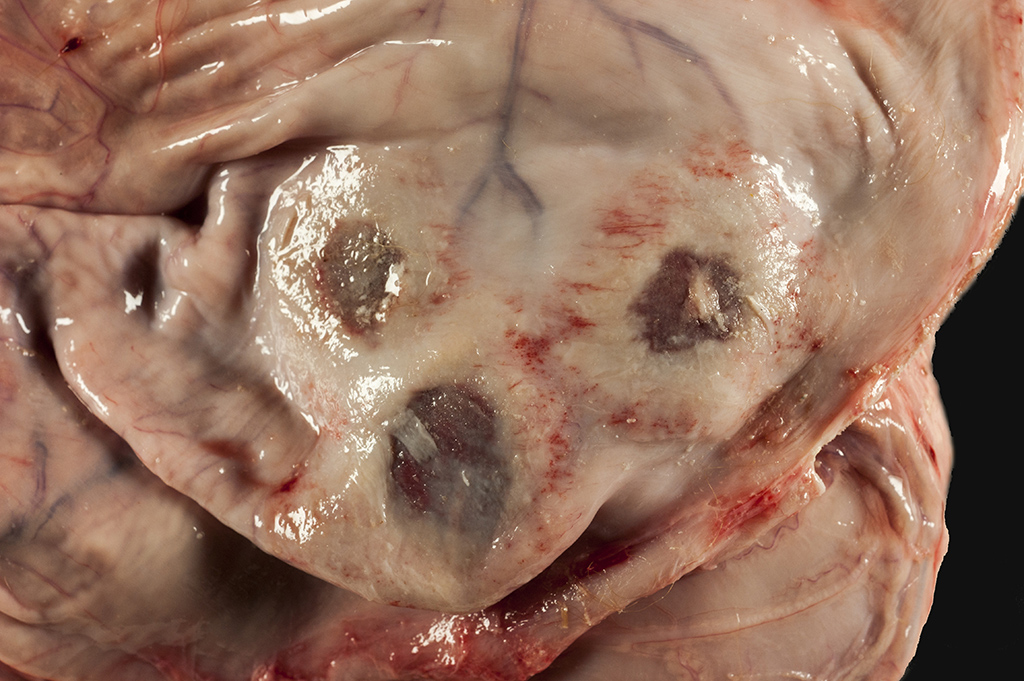
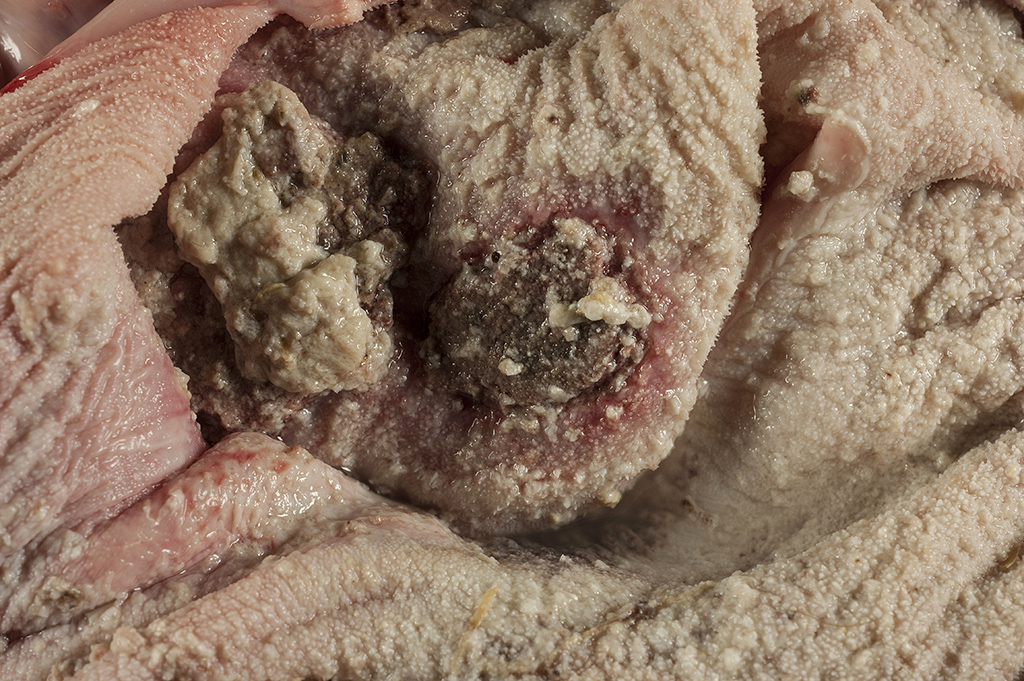
Gross Pathology: The calf is in poor body condition and in a fresh state of preservation. The abomasum contains multiple 1-2mm brown/red foci on the mucosal surface and the luminal contents are green-yellow and watery. Several discrete red foci (10-15cm diameter) with fibrin tags are visible on the serosal surface of the rumen. These lesions are transmural and correspond with friable, brown and tan plaques surrounded by a hyperemic border on the mucosal surface. Approximately 70% of the intervening ruminal mucosa is grey and mildly thickened. The rumen is filled with milky fluid and clots and has a pH of 5. Multiple serosal segments of the small and large intestine are reddened.
Laboratory results: None provided.
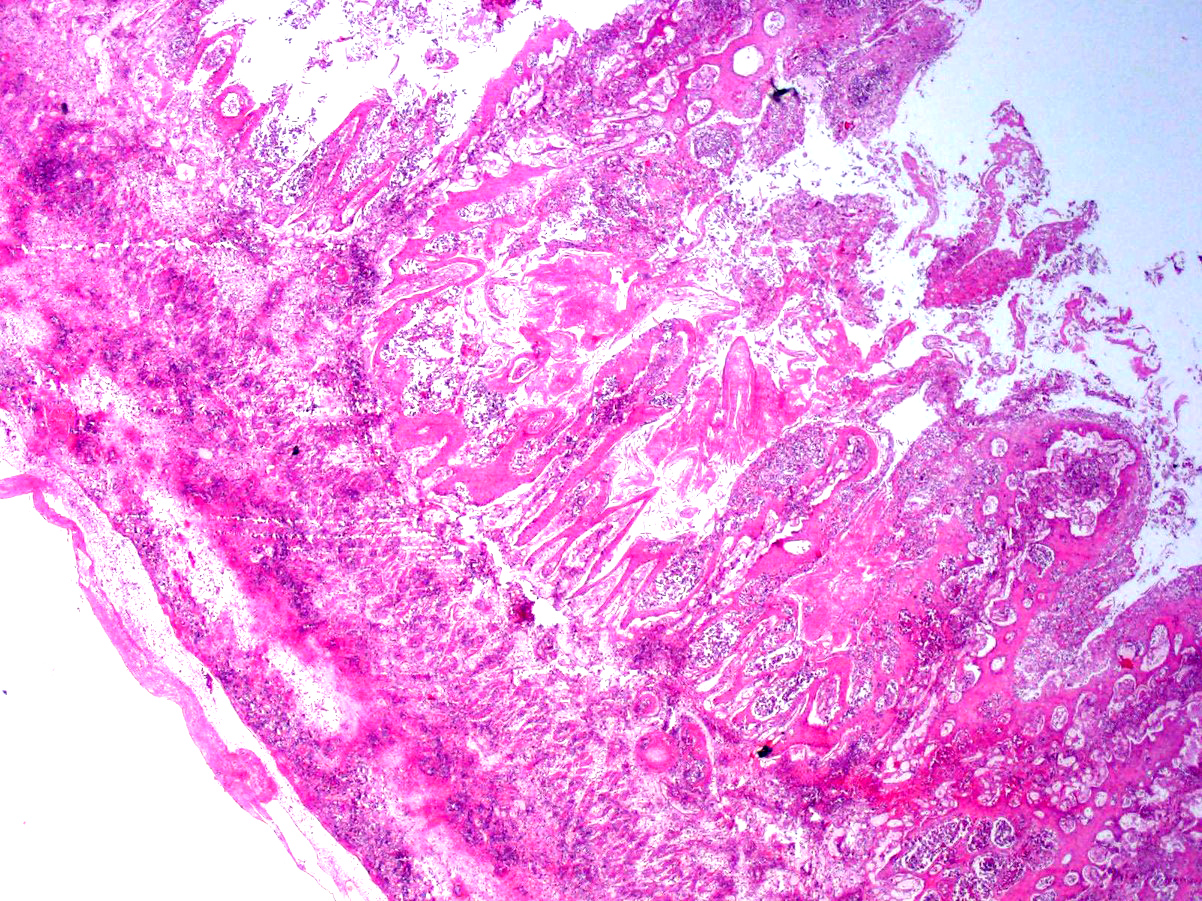
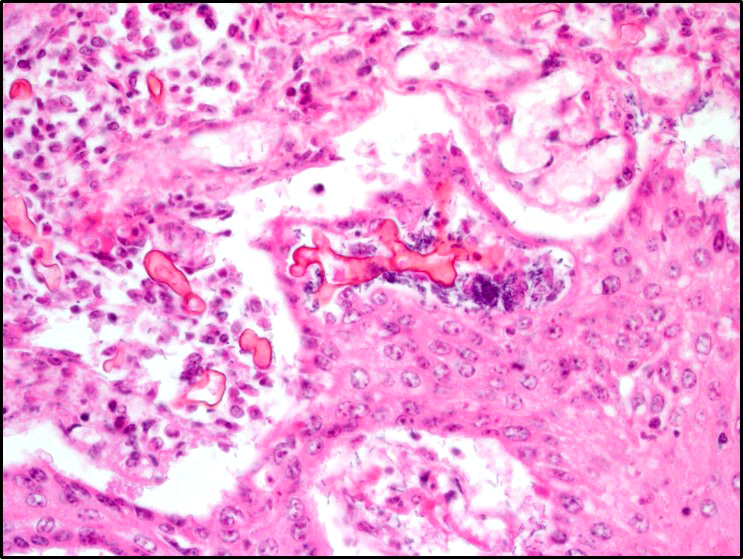
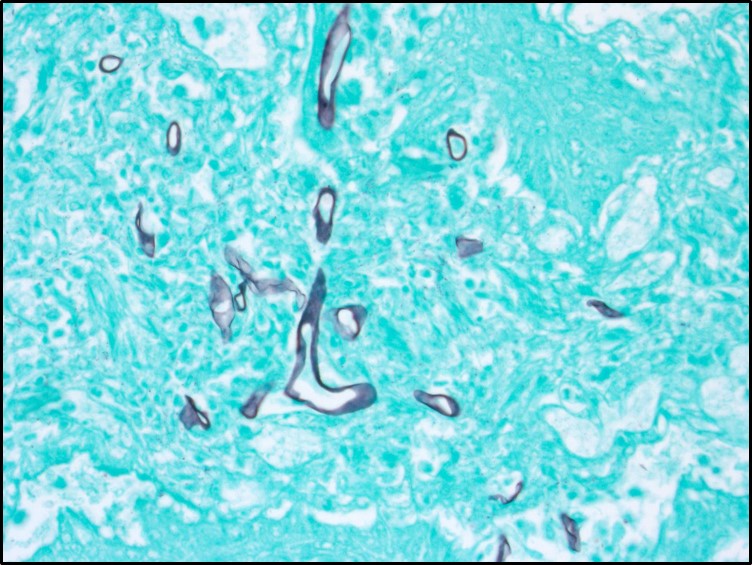
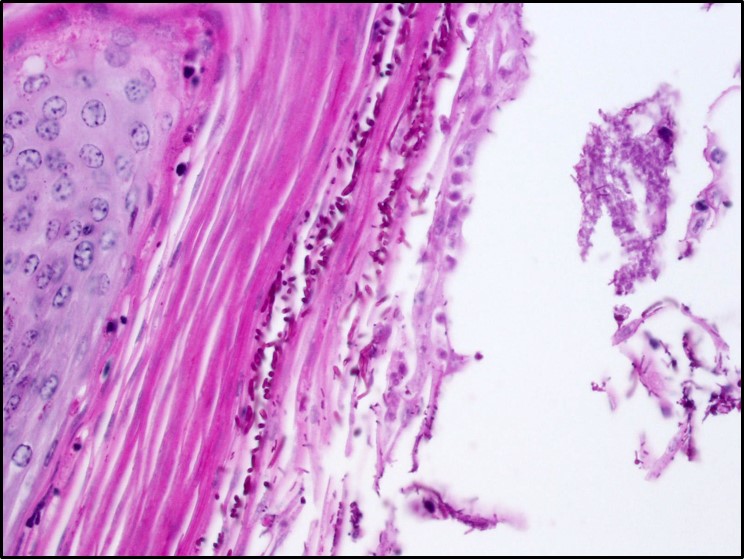
Microscopic Description: Rumen: focally and transmurally, the rumen wall is markedly expanded by edema, fibrin and eosinophilic karyorrhectic debris intermixed with numerous inflammatory cells that consist predominantly of intact and degenerate neutrophils, macrophages and smaller numbers of lymphocytes and plasma cells. Multifocally and affecting the serosa and submucosa adjacent the necrotic lesion, there are large numbers of mesenchymal cells admixed with thin strands of collagen. Multiple submucosal blood vessels contain fibrin thrombi and are hyalinized and disrupted by moderate numbers of neutrophils and fungal hyphae that are 5-20um wide, pauci-septate, have thin, non-parallel walls, exhibit irregular, non-dichotomous or right-angled branching and occasionally contain focal bulbous dilatations. Moderate numbers of fungal hyphae are present within the mucosa, submucosa and extending to the serosa. Multifocally, there is mild to moderate hyperkeratosis, erosion and ulceration of the squamous epithelium and there are numerous intraepithelial aggregates of neutrophils (microabscesses). Multifocally between keratin lamellae there are small numbers of basophilic, 2-4um diameter, round to oval and occasionally pseudoseptate fungal organisms. Multifocally there are large numbers of purple bacterial colonies within the superficial mucosa.
Contributor’s Morphologic Diagnosis:
Rumen: severe, chronic, focally extensive, necrotizing rumenitis with vasculitis and thrombosis and intralesional fungal hyphae morphologically consistent with zygomycetes.
Moderate, chronic, locally extensive ruminal hyperkeratosis with intralesional fungal organisms morphologically consistent with Candida.
(Mycotic rumenitis)
Contributor’s Comment: Opportunistic fungal organisms such as the zygomycetes of the genera Mucor, Absidia and Rhizopus, Aspergillus, and occasionally Candida can cause mycotic rumenitis in calves. The two fungi involved in this case, the zygomycetes and Candida require predisposing circumstances such as stress, sepsis, long-term administration of antibiotics or ruminal acidosis to cause disease.2,9 The zygomycetes are saprophytic and ubiquitous organisms found in the environment, and are common gastrointestinal inhabitants of animals. They are angioinvasive and gain access to the vascular system through injury to mucosal barriers. The yeast Candida, in which C. albicans is most often implicated in animal disease, is a common inhabitant of the gastrointestinal tract, genital mucosa and upper respiratory tract of mammals. Candida exists as a yeast form but can undergo a phenotypic switch to the invasive, filamentous form when there are perturbations to the mucosa or physiological state of an animal.11 Neither organism should cause disease in an immunocompetent animal.
The incidence of mycotic infections in the forestomach of calves diagnosed at necropsy is relatively low. The incidence rate of alimentary mycosis in calves less than 6-months-old presented to necropsy over a 10-year period is 3%.4 The most common organisms isolated are the zygomycetes, followed closely by Aspergillus. Co-infection, where both organisms are identified, is also reported.4,8 Isolation of Candida is uncommon; however, it is reported to occur in co-infections in adjacent, non-inflamed regions of the mucosa.8 The most common risk factor for mycotic infection of the forestomachs in these calves is diarrhea that is treated with antibiotics.
The pH of the ruminal fluid in this calf was 5, which is below normal physiological levels in a milk-fed calf (6.4-7.0).5 Ruminal acidosis in pre-ruminant calves is primarily caused by esophageal groove dysfunction that results in the spillage and subsequent stagnation and putrefaction of milk in the rumen and reticulum.6 Risk factors associated with esophageal groove dysfunction include the feeding of milk from a pail or tube, and neonatal diarrhea.5 Ruminal acidosis causes mild mucosal inflammation, and coupled with the other precipitating factors in this case, such as the antibiotic use and stress, precipitated a high-risk environment for opportunistic fungal infection.
JPC Diagnosis: Rumen: Rumenitis, necrotizing, focally extensive, severe with necrotizing vasculitis, widespread vascular thrombosis, and numerous fungal hyphae.
Conference Comment: Distinguishing the ruminant forestomachs histologically can be daunting, particularly with advanced disease. Here are a few key features that may simplify the process.
The rumen contains small tongue-shaped papillae which become more substantial as the animal is weaned and begins eating more roughage. The mucosal epithelium has three functions: protection, metabolism, and absorption and is keratinized to protect against rough, fibrous ingesta. Within the deeper strata, short chain volatile fatty acids are metabolized (butyric, acetic, and proprionic acids). Microscopically, what sets the rumen apart is the shape of the papillae and the lack of lamina muscularis extension into the papillae.1
Next, the reticulum is composed of long, thin primary lamellae with knob-like projections extending off termed secondary lamellae and permanent interconnecting folds (reticular crests) giving the gross appearance of a honeycomb. The reticulum is also lined by keratinized stratified squamous epithelium and the key microscopic feature are small slits of lamina muscularis within the tips of the primary lamellae.1
Finally, the omasum has even more folded lamellae which extend like pages of a book grossly and are lined by keratinized stratified squamous epithelium, all be it not as keratinized as the ruminal papillae. In the omasum, form follows function, and there is a much thicker lamina muscularis beneath the lamina propria that extends into the entire length of the lamellae providing the ability to squeeze ingesta into the abomasum.1
Both Candida spp. and zygomycetes are normal flora in the ruminant gastrointestinal tract.
Zygomycosis comprises numerous etiologic agents which can manifest clinically as cutaneous, subcutaneous, systemic (pulmonary or gastrointestinal), or rhinocerebral infections. The microscopic hallmark of all zygomycetes is the invasive mycelial form in tissues which is infrequently septate and exhibits non-dichotomous branching with non-parallel walls and rare bulbous dilatations. The hyphae are often surrounded by eosinophilic granular material (Splendore-Hoeppli phenomenon) and angioinvasion is common particularly with Mucorales. The main microscopic differential for zygomycosis is pythiosis and must be distinguished using immunohistochemistry, culture, or PCR. In addition to mycotic rumenitis, zygomycetes are a common cause of abortion in ruminants and a differential for granulomatous lymphadenitis.3,7,11
In contrast with zygomycosis, Candida spp. are less invasive and often associated with prolonged antibiotic use, immunosuppression, concurrent disease, or stress. Anorexia in chronically ill animals results in increased keratin along the surface of mucus membranes which may provide a fertile environment for Candida to hang on and grow. An important determinant of virulence amongst Candida spp. is the presence of adhesins which aide in binding to host tissues. Specifically, yeast bind mannose receptors and hyphae bind complement receptor 3 (CR3) and the Fc-gamma receptor. Adherence and resistance to antifungal drugs is facilitated by biofilm formation. Additionally, Candida species produce enzymes to degrade proteins in the extracellular matrix which aides in dissemination (aspartyl proteinases), resist oxidative killing by phagocytes (catalase), and block neutrophil degranulation (adenosine). Microscopically, Candida spp. Are seen in three, often comingling, forms: pseudohyphae, hyphae, and yeast. The pseudohyphal form is composed of chains of blastoconidia and distinguished from the hyphal form by narrowed points of attachment of adjacent yeast. The yeast form can often be seen reproducing by narrow-based budding.3,10,11
Conference attendees viewed PAS, GMS, and PTAH immunohistochemical stains to identify organisms and fibrin thrombi present in the section. Polymerized fibrin was identified first at the periphery of affected vessels, plugging endothelial holes, and eventually filling the lumen. While the pathogenicity of the fungal hypehae, present transmurally and often within the walls of necrotic vessels were in doubt, the damage resulting from presumptive yeast forms of Candida present largely in the hyperkeratotic layer and mucosa was a source of spirited discussion.
In addition, the possibility of this calf as a “ruminal drinker” (a calf with abnormal closure of the rumenoreticular groove during nursing, which directs milk directly into the stomach) was discussed, the history of tube-feeding was also cited as a possibility for the introduction of milk into the developing rumen, where it might be used as a culture medium for a range of commensal bacterial and fungal agent.
Contributing Institution:
http://ivabs.massey.ac.nz
http://vet-school.massey.ac.nz
References:
- Bacha WJ, Bacha LM. Color Atlas of Veterinary Histology. 3rd ed. Ames, IA: Wiley-Blackwell; 2012:155-157.
- Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 5th London, UK: Saunders; 2007:1-296.
- Chandler FW, Kaplan W, Ajello L. Color Atlas and Text of the Histopathology of Mycotic Diseases. Chicago, IL: Year Book Medical Publishers, Inc.; 1980:42-45, 122-126.
- Chihaya Y, Furusawa Y, Okada H, Matsukawa K, Matsui Y. Pathological studies on systemic mycoses in calves. Journal of Veterinary Medical Science. 1991; 53(6):1051-1058.
- Dirksen G, Dirr L. Oesophageal groove dysfunction as a complication of neonatal diarrhea in the calf. Bovine Practitioner. 1989; 17(4):353-358.
- Gentile A. Ruminal acidosis in milk-fed calves. Large Animal Veterinary Rounds. 2004:4(9).
- Ginn PE, Mansell JEKL, Rakich PM. Integumentary system. ln: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol 1. 6th ed. Philadelphia, PA: Elsevier Saunders. 2016:659-660.
- Neitzke JP, Schiefer B. Incidence of mycotic gastritis in calves up to 30 days of age. Canadian Veterinary Journal-Revue Veterinaire Canadienne. 1974; 15(5):139-143.
- Quinn PJ, Markey BK. Mycology. In: Concise Review of Veterinary Microbiology. 1st London, UK: Wiley-Blackwell; 2003:70-87.
- Uzal FA, Plattner BL, Hostetter JM. Alimentary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol 2. 6th ed. Philadelphia, PA: Elsevier; 2016:202.
- Zachary JF. Mechanisms of microbial infection. In: Zachary JF, McGavin MD, eds. Pathologic Basis of Veterinary Disease. 5th St. Louis, MO: Elsevier; 2011:147-241.