WSC 2023-2024, Conference 25, Case 1
Signalment:
Female goat (Capra hircus), age and breed unspecified.
History:
The owners had 3 goat does that aborted premature and full-term fetuses after being purchased 4 months prior. These goats were dewormed a week before sample submission. Submitted for necropsy evaluation are 2 fetuses and one placenta.
Gross Pathology:
No major gross lesions were observed in the placenta.
Laboratory Results:
Q-fever PCR at referral laboratory – Positive for Coxiella burnetti.
Microscopic Description:
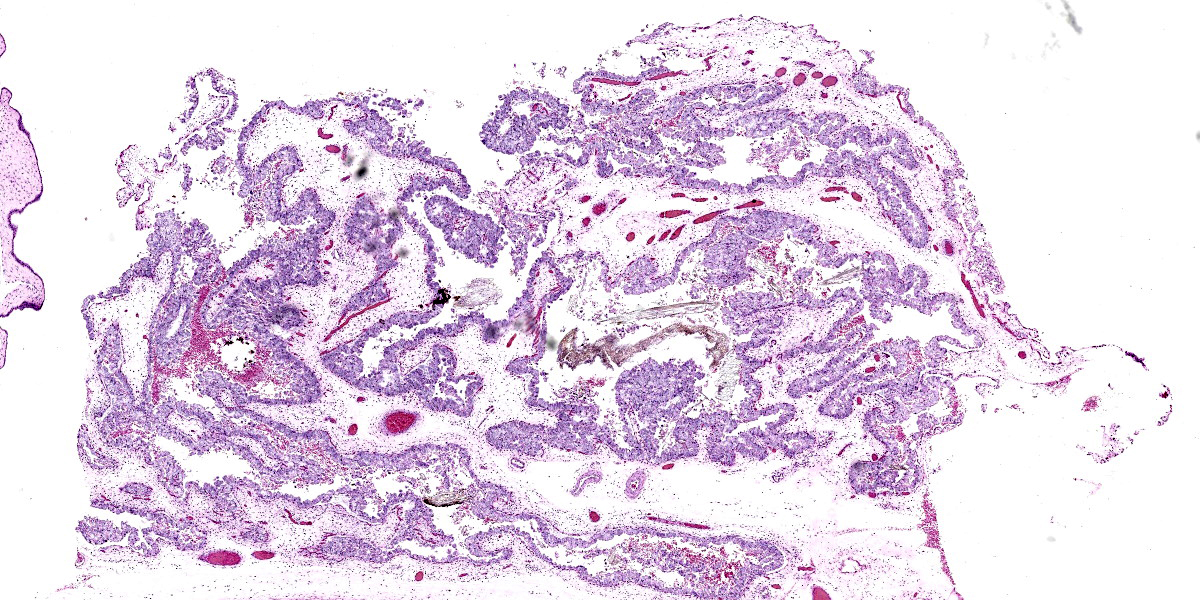
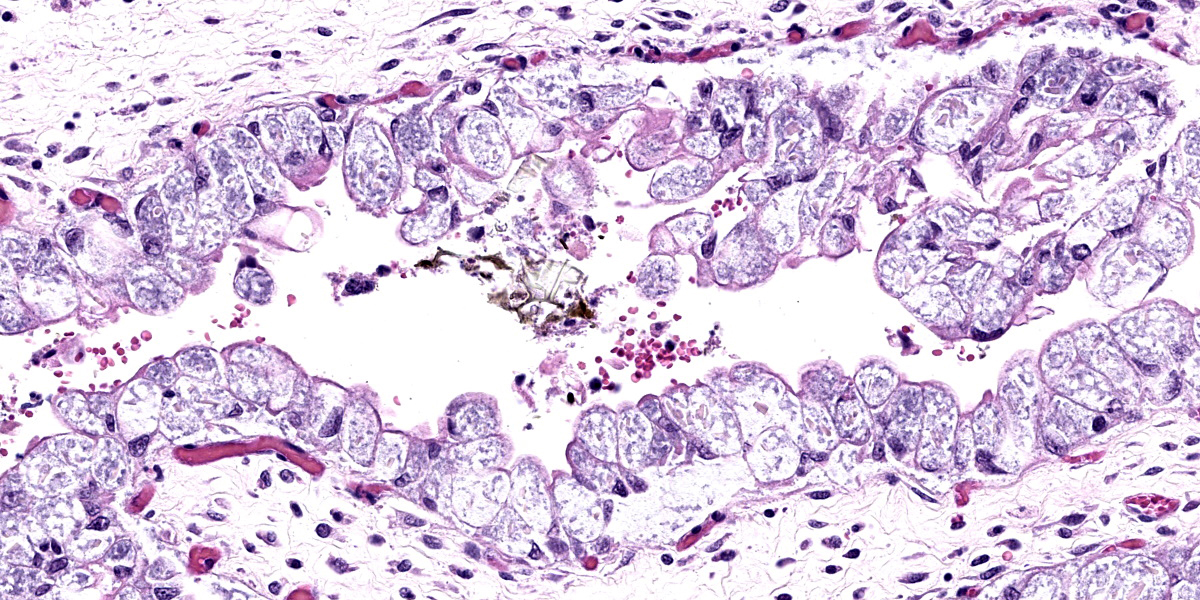
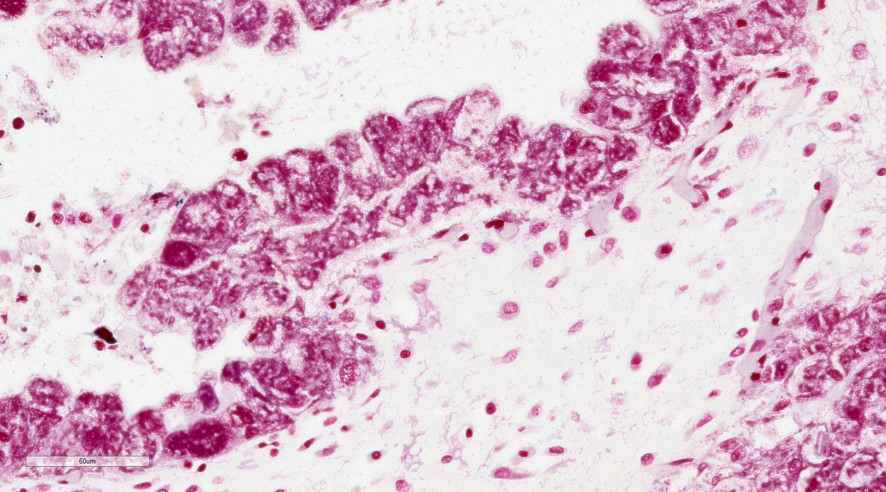
Placenta (chorioallantois): In regionally extensive areas, numerous intracytoplasmic pleomorphic (cocci, coccobacillary, and bacillary morphologies), gram negative bacterial microorganisms are observed within hypertrophied syncytiotrophoblasts on the chorionic villi. The chorioallantois is severely expanded by edema and numerous inflammatory infiltrates, predominantly composed of plasma cells, lymphocytes and hofbauer cells (placental macrophages). Small caliber vessels in the chorionic stroma are moderately dilated by congestion.
Contributor’s Morphologic Diagnosis:
Placenta: Placentitis, lymphoplasmacytic and histiocytic, acute to subacute, regionally extensive, severe with intratrophoblastic bacteria (Coxiella burnetti).
Contributor’s Comment:
Q fever, the disease caused by the bacterial organism Coxiella burnetti, an obligate intracellular pleomorphic gram-negative bacterial microorganism, is considered a potential bioterrorism agent in many countries, including the United States. A worldwide presence has been reported, except in New Zealand.
The US Centers For Disease Control has classified C. burnetti as ‘category B’ – moderately easy to disseminate with moderate morbidity and mortality. Previous findings indicate that C. burnetti has a low infectious dose for initiating a disease process i.e., up to 90% probability for a single bacterium to initiate a disease process.9
Coxiella spp. belong to class Gammaproteobacteria, order Legionellales and family Coxiellaceae. C. burnetti has been identified in multiple species including domestic ruminants, birds, and some insects.2,6,11,16 Domestic ruminants are frequently considered as reservoir hosts for C. burnetti.7,13 This pathogen is zoonotic to humans, and can cause a wide array of clinical findings and lesions in different species, that includes abortions, stillbirths, hepatomegaly, splenomegaly, endocarditis, and encephalitis.2
C. burnetti has been identified in the gut cells of ticks (Dermacentor sp., Haemaphysalis sp., Ixodes sp., Hyalomma sp., Ornithodoros sp.,) implicating these ticks as one of the major modes of transmission for this pathogen.3 Other recorded routes of transmission include aerosolization, ingestion of contaminated placenta (in dogs and cats), exposure to infected milk and other dairy products, horizontal (person-to-person), exposure to fomites, and contact with infected hide or wool from animals.1 Transmission via fomites, reproductive tissues, and contaminated, unpasteurized dairy products are the most prominent causes for pathogen transmission across different species.
A biphasic growth pattern has been reported for this obligate intracellular gram-negative microorganism, characterized by two major cell types – a spore-like small cell variant (SCV) and an actively dividing large cell variant (LCV).2,7,12,14 This spore-like SCV has a higher concentration of peptidoglycan, providing resistance to a wide array of physical and chemical stressors, thus permitting the pathogen to survive in the environment. SCVs are usually shed into the environment via placental fluids, mammary secretions, urine, feces, and fomites during the parturition process. By inhalation and close contact, these SCVs then spread to others in the immediate vicinity. Following inhalation, C. burnetti is commonly observed within macrophages in various organs including lungs, which leads to inhibition of cell death by impairing phagolysosome formation and macrophage function.12,14 The exact mechanism for tropism towards trophoblasts is still definitively undetermined, although it is believed that mild immunosuppression during pregnancy may be one of the causes for pathogen localization in the placenta.18 Maturation and development of SCV in the placenta following a biologically active LCV phase will lead to further spread of the pathogen through shedding into the environment.
In humans, C. burnetti can result in the following: self-limiting flu-like clinical signs, severe atypical pneumonia, hepatitis with severe hepatomegaly and icterus, maculopapular exanthema, pericarditis, myocarditis, aseptic meningitis, seizures, polyradiculoneuritis, optic neuritis, transient hypoplastic anemia, and/or lymphadenopathy, as well as other manifestations.8 The chronic form of Qfever has been reported in 5% of all clinical reports and the most commonly reported lesions in chronic disease are endocarditis, hepatic fibrosis, osteoarthritis, osteomyelitis, as well as other manifestations.8
One previous study has reported strong placental tropism of C. burnetti at 2-4 weeks after inoculation in pregnant goats, and higher numbers of microorganisms isolated from these infected goats at the time of kidding.15 In domestic ruminants, histological findings from reproductive tissues (such as the placenta) are a histiocytic and lymphoplasmacytic placentitis with numerous intrahistiocytic and intratrophoblastic bacterial microorganisms. Other commonly used diagnostic techniques includes immunoperoxidase staining for highlighting bacterial microorganisms, immunohistochemistry using monoclonal antibodies against C. burnetti, PCR amplification of different DNA targets including 16S and 23S, and serological techniques such as ELISA, complement fixation, western blotting, dot blotting, and microagglutination.8
Q-fever is still poorly understood and is zoonotically important due to its multiple attributes, including low infectious dose and environmental persistence. Subsequent screening of all abortions in domestic ruminants, decontamination, biosecurity and treatment are strongly recommended to curb the spread of this pathogen.
The main findings in this case are: 1) lymphoplasmacytic and histiocytic placentitis, 2) the presence of large numbers of intratrophoblastic gram negative bacterial organisms, and 3) PCR positive detection of C. burnetti. Altogether, these findings are consistent with Coxiella burnetti infection, resulting in abortion in this case.
Contributing Institution:
Oklahoma State University
Department of Veterinary Pathobiology
College of Veterinary Medicine
Stillwater, OK 74078 USA
www.vetmed.okstate.edu
JPC Diagnosis:
Placenta: Placentitis, lymphohistiocytic, diffuse, mild, with innumerable intratrophoblastic coccobacilli.
JPC Comment:
Coxiella burnetii, like any self-respecting intracellular pathogen, must find a way to solve an existential problem: how to survive inside a cell that is trying to kill it. C. burnetii employs a variety of weapons to this end, some of which are well-characterized, and some of which remain poorly understood.
A key weapon in its arsenal is a Type IV secretion system (T4SS), one of several types of such systems used by microorganisms to transport macromolecules across cell membranes.17 Once C. burnetti is phagocytosed by a macrophage, it uses its T4SS to modify host cellular processes to develop a C. burnetti-containing vacuole (CCV) in which it can thrive and replicate. The CCV is characterized by “promiscuous fusogenicity,” prominent size, temporal stability, and the ability to promote C. burnetti replication, all of which distinguish the CCV from a typical lysosome.10 The T4SS is critical to the formation and maintenance of this CCV intracellular niche and to thwarting the host cell death pathways which are typically arrayed against it.4
The C. burnetii T4SS transfers over 130 bacterial proteins, very few of which have been characterized, from the CCV into the macrophage cytoplasm.4 Among those bacterial proteins with known functions, one, AnkG, binds mitochondrial p32 which inhibits the intrinsic apoptosis pathway. Another, inhibitor of caspase activation (IcaA), frustrates pyroptosis by inhibiting the NLRP3 inflammasome and caspase-1 activation.4
The effects of translocated bacterial proteins can be appreciated, even if the mechanism of action or the specific protein cannot be identified. One such example relates to the nutrient-sensing mammalian target of rapamycin complex 1 (mTORC1) which homeostatically inhibits autophagy but is itself inhibited in times of nutrient deprivation. Inhibition of mTORC1 typically leads to autophagy, the catabolism of cellular organelles and the repurposing of the liberated macromolecules as metabolic substrates. Inhibition of MTORC1 leads to the formation of large, fusogenic lysosomal organelles that anticipate the surge of incoming catabolic cargo.10
Research has demonstrated that C. burnetti inhibits mTORC1 by an unknown mechanism in the face of nutrient sufficiency, leading to the development of the large, fusogenic CCV that accommodates hundreds of replicative organisms.10 The CCV fuses with autophagic vesicles and accumulates autophagy-related proteins such as beclin-1, LC3, and p62. The critical role of T4SS in this process, and by extension, the critical role of translocated bacterial proteins, is evidenced by experimental studies that disrupt T4SS function and inhibit nutrient-dependent autophagy and bacterial replication in C. burnetti infected cells.10 Researchers have speculated that C. burnetti activates autophagic catabolism via mTORC1 inhibition to provide replication-supporting nutrients within the CCV.10
C. burnetti likely has many more undiscovered virulence factors which are the subject of active research due to its highly infectious nature and unique mechanisms of intracellular survival. A nice summary of the current understanding of C. burnetti virulence factors can be found in a 2020 review by Dragan and Voth.5
Our moderator this week was Dr. Susan Bender, Assistant Professor of Clinical Pathobiology ar the University of Pennsylvania School of Veterinary Medicine. Participants seemed to approach this slide with some trepidation and initial discussion focused on the basics of tissue identification and proper terminology. Dr. Bender noted that the challenge was made even more difficult by the tremendous distention of the trophoblasts by Coxiella organisms.
Participants also discussed an area of the tissue that appeared to contain squamous metaplasia, which provoked some discussion as this would be an unusual finding in the chorioallantois (though not unprecedented; this change can be observed in fungal abortions in goats). Dr. Bender noted that these most likely represent amniotic plaques, a normal finding in the amnion, a portion of which is likely in the slide along with the chorioallantois. A few participants also noted possible thrombi in a a few vessels; however, these are likely not significant without accompanying vascular injury, which is not seen in section and is not an expected finding for this disease entity.
The remarkable expansion of the trophoblasts in this case is a key histologic feature, which Dr. Bender noted should bring a differential list to mind. While the blue frothy material is a classic appearance for Coxiella burnetii, Chlamydia abortus should also be on the differential list. Other differentials include Brucella spp., Listeria spp., and Campylobacter spp., though those agents do not typically cause such massive trophoblast expansion. Dr. Bender ended discussion by reminding participants that Q fever is a reportable diseaes which, if encountered, should be promptly reported to state authorities with no bleating around the bush!
Participants noted the severe inflammation described by the contributor; however, in the section assessed in conference, participants felt the inflammation was rather mild and predominantly lymphohistiocytic, leading to some minor tinkering with the morphologic diagnosis.
References:
- Angelakis E, Raoult D. Q fever. Vet Microbiol. 2010;140(3-4):297-309.
- Van den Brom R, van Engelen E, Roest HIJ, van der Hoek W, Vellema P. Coxiella burnetii infections in sheep or goats: an opinionated review. Vet Microbiol. 2015; 181(1-2):119-129.
- Celina SS, Cerný J. Coxiella burnetii in ticks, livestock, pets and wildlife: A mini-review. Front Vet Sci. 2022;9:1068129.
- Delaney MA, den Hartigh A, Carpentier SJ, et al. Avoidance of the NLRP3 inflammasome by the stealth pathogen, Coxiella burnetii. Vet Pathol. 2020;58 (4):624-642.
- Dragan AL, Voth DE. Coxiella burnetii: international pathogen of mystery. Microbes Infect. 2020;22(3):100-110.
- Ebani VV, Mancianti F. Potential role of birds in the epidemiology of Coxiella burnetii, Coxiella-like agents and Hepatozoon spp. Pathogens. 2022;11(3):298.
- Eldin C, Mélenotte C, Mediannikov O, et al. From Q fever to Coxiella burnetii infection: A paradigm change. Clin Microbiol Rev. 2017;30(1):115-190.
- Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36(7):1823-1834.
- Jones RM, Nicas M, Hubbard AE, Reingold AL. The Infectious Dose of Coxiella Burnetii (Q Fever). Appl Biosaf. 2006;11(1):149-156.
- Larson CL, Sandoz KM, Cockrell DC, Heinzen RA. Noncanonical inhibition of mTORC1 by Coxiella burnetii promotes replication within a phagolysosome-like vacuole. mBio. 2019;10(1):e02816-18.
- Mccaughey C, Murray LJ, Mckenna JP, et al. Coxiella burnetii (Q fever) seroprevalence in cattle. Epidemiol Infect. 2010; 138(1):21-27.
- McCaul TF, Williams JC. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol. 1981; 147(3):1063.
- Miller JD, Shaw EI, Thompson HA. Coxiella burnetii, Q Fever, and Bioterrorism. In: Anderson B, Friedman H, Bendinelli M, eds. Microorganisms and Bioterrorism. Springer;2006:181-208.
- Minnick MF, Raghavan R. Developmental biology of Coxiella burnetii. Adv Exp Med Biol. 2012;984:231-248.
- Roest HJ, van Gelderen B, Dinkla A, et al. Q Fever in Pregnant Goats: Pathogenesis and Excretion of Coxiella burnetii. PLoS One. 2012;7(11):e48949.
- Tokarevich NK, Panferova YA, Freylikhman OA, et al. Coxiella burnetii in ticks and wild birds. Ticks Tick Borne Dis. 2019;10(2):377-385.
- Wallden K, Rivera-Calzada A, Waksman G. Type IV secretion systems: versatility and diversity in function. Cell Microbiol. 2010;12(9):1203-1212.
- Zarza SM, Mezouar S, Mege JL. From Coxiella burnetii Infection to Pregnancy Complications: Key Role of the Immune Response of Placental Cells. Pathogens. 2021;10(5):627.