CASE I: S788/08 (JPC 3102484).
Signalment: 1.2-year-old female common squirrel monkey, Saimiri sciureus, New World monkey.
History: The animal showed severe dyspnea, apathy, hypothermia and mucous nasal discharge. In the oral cavity a mucous exudate and multifocal moderate gingival erosions were observed. The animal?s condition worsened gradually and i t died spontaneously.
Gross Pathology: At necropsy the body was in a moderate nutritional condition. In the oral cavity multifocal gingival ulcerations of 1 to 2 mm in diameter were observed. The lung showed severe congestion, moderate alveolar edema and multifocal hemorrhages of 2 to 4 mm in diameter. The spleen was moderately enlarged. The liver displayed moderate diffuse lipidosis. On the left shoulder (at the level of the supraspinatus muscle) a subcutaneous hemorrhage, 2 x 2 cm, was observed.
Laboratory Results:
Radiological findings: mild multifocal radiodense areas in the thoracic cavity.
Using an antibody against human Herpes simplex virus type 1, a strong positive reaction (Herpes simplex virus infection) was observed in tissue sections of the oral mucosa and liver.
Ultrastructurally, in hepatocytes intranuclear herpesviral nucleocapsids were observed.
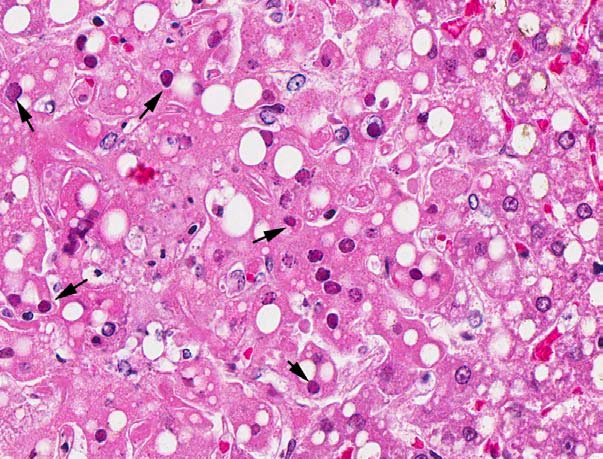
Microscopic Description: The liver had a regular architecture. There were irregularly distributed, sublobular foci of coagulation necroses. Hepatocytes adjacent to the necrotic areas displayed cytoplasmic vacuoles of variable size interpreted as fatty degeneration. Furthermore, in some perilesional hepatocytes large eosinophilic intranuclear inclusion bodies causing chromatin margination and clumping were observed. The liver also displayed a mild to moderate, acute congestion.
Contributor Morphologic Diagnosis: Liver: multifocal moderate acute coagulation necrosis with eosinophilic intranuclear inclusion bodies, and multifocal moderate hepatic lipidosis.
Contributor?s Comment: In the submitted liver tissue, the main lesion consists of multifocal coagulation necrosis, and eosinophilic intranuclear inclusion bodies in adjacent hepatocytes.
Upon histological examination of other tissues, a severe multifocal ulcerative to necrotizing gingivitis with hydropic degeneration of epithelial cells, multinucleated giant cells (syncytia), eosinophilic intranuclear inclusion bodies, and a moderate necrotizing vasculitis was observed. The cerebellum showed a moderate subacute multifocal necrotizing inflammation with eosinophilic intranuclear inclusion bodies in neurons. The small intestine displayed a subacute multifocal moderate necrotizing enteritis with multinucleated cells (syncytia) and intranuclear eosinophilic inclusion bodies in enterocytes.
In the spiral ganglion of the ear, numerous eosinophilic intranuclear inclusion bodies were observed. The lymphoid tissues showed a severe lymphocytic depletion. In the nasal cavity a mild subacute diffuse purulent inflammation was present. In the lung, a mild multifocal subacute histiocytic pneumonia, moderate fibrin-rich alveolar edema, severe multifocal hemorrhages and severe congestion were found.
The detection of syncytia and eosinophilic intranuclear inclusion bodies as well as the ultrastructural demonstration of herpesviral nucleocapsids indicates a s y s t e m i c h e r p e s v i r a l i n f e c t i o n . U s i n g immunohistochemistry an infection with human Herpes virus type 1 (HHV-1; Herpes simplex virus 1) was confirmed. Based on the ulcerative to necrotizing lesions, syncytia formation and the detection of eosinophilic intranuclear inclusion bodies in the mouth of the affected animal, an oral route of infection should be considered.
Nonhuman primates are primary hosts of a n u m b e r o f a l p h a - a n d gammaherpesviruses, whereas some host specific herpesviruses (e.g. Herpesvirus ateles, Herpesvirus saimiri, Rhesus rhadinovirus) were described.2,8,11 In their natural hosts these viruses cause mild or inapparent infections, but they generally are associated with severe infections when interspecies transmission occurs.3,4,10 The ability of herpesviruses to cross interspecies barriers is responsible for a major zoonotic risk of these pathogens.5,8 Hence, the human herpesviruses are transmissible from h u m a n s t o p r i m a t e s ; h o w e v e r , spontaneous infections in monkeys appear to be rare.5,8 In Old World primates, HHV-1 infections remain localized at the mucocutaneous tissues and the virus-host relationship is comparable to that of humans.6,8 In contrast, New World monkeys and prosimians are more susceptible to infection and systemic disease.5
Herpesviruses are a family of large DNA viruses which infect humans, mammals, vertebrates and invertebrates.1,9 The virions are 200-250 nm in diameter, consist of a linear double-stranded DNA genome of 120-240 kbp packaged in an icosahedral capsid approximately 125 nm in diameter, embedded in a matrix containing many viral proteins, itself wrapped in a lipid membrane containing several glycoproteins.2,9 The family Herpesviridae is divided i n t h r e e s u b f a m i l i e s A l p h a - , B e t a - a n d Gammaherpesvirinae on the basis of their genomic attributes.2,9
In spontaneously infected owl monkeys, (Aotus trivirgatus), tree shrews (Tupaia glis), common marmosets (Callithrix jacchus), black-tufted-ear marmosets (Callithrix penicillata) and lemurs, a severe disease leading to death occurs in most cases.8 In most mentioned species, erosions and ulcers of the oral mucous membranes and mucocutaneous junction of the lips, and focal necrosis and hemorrhage and eosinophilic intranuclear inclusion bodies were observed.5,8 The source of the herpesvirus infection in nonhuman primates seems to be, in most cases, the close contact with persons shedding virions.8 Close contact is also necessary for the transmission of the infection, hence most cases of herpesvirus infections in nonhuman primates occur in animals kept by private persons.8 Therefore, the use of appropriate protective measures for humans handling nonhuman primates should greatly reduce the risk of infection.
JPC Diagnosis: 1. Liver: Hepatitis, necrotizing, multifocal and random, mild, with hepatocellular intranuclear viral inclusion bodies.
2. Liver, hepatocytes: Lipidosis, micro- and macrovesicular, diffuse, moderate.
JPC Comment: Herpes simplex (Herpesvirus hominis) has two distinct subtypes. Herpes simplex type 1 (HSV-1) causes oral lesions and encephalitis in adult humans, and HSV-2 causes genital lesions in adult humans and systemic disease in human infants. Both types produce the same fatal disease in New World monkeys.
Discussion during the conference was focused on developing a plausible differential diagnosis for these lesions in both New and Old World monkeys: Macaqacine herpesvirus (herpesvirus B), which causes fatal encephalomyelitis in man, produces similar lesions as seen in this case of necrotizing hepatitis as well as hemorrhagic necrosis in the lung, brain, and lymphoid organs. Herpesvirus tamarinus (herpesvirus T), which is asymptomatically carried by squirrel monkeys, and herpes simplex both cause identical necrotizing lesions in other New World monkeys such as aotus monkeys, marmosets, and tamarins. Virus isolation or immunohistochemical staining is necessary to differentiate them. Simian varicella virus causes similar necrotizing lesions with herpetic viral inclusions and syncitial cells of vesicular rash and encephalitis, pneumonia, and hepatitis in African green monkeys and other Old World monkeys.7
Contributor: University of Veterinary Medicine, Hannover
Department of Pathology Bünteweg 17
D-30559, Hannover Germany
http://www.tiho-hannover.de/einricht/patho/index.htm
References:
1. Batista FM, Arzul I, Pepin JF, et al. Detection of ostreid herpesvirus 1 DNA by PCR in bivalve molluscs: a critical review. J. Virol. Methods. 2007;139 (1):1-11.
2. Davison AJ, Trus BL, Cheng N, et al. A novel class of herpesvirus with bivalve hosts. J Gen Virol. 2005;86,41-53.
3. Fickenscher H, Fleckenstein B. Herpesvirus saimiri. Philos. Trans. R . Soc. Lond. B Biol. Sci. 2001;356 (1408):545-567.
4. Hall KT, Giles MS, Goodwin DJ, et al. Characterization of the herpesvirus saimiri ORF73 gene product. J. Gen. Virol. 2000;81(Pt 11):2653-2658.
5. Juan-Sallés C, Ramos-Vara JA, Prats N, et al. Spontaneous herpes simplex virus infection in common marmosets (Callithrix jacchus). J. Vet. Diagn. Invest. 1997;9(3):341-345.
6. Kik MJ, Bos JH, Groen J, et al. Herpes simplex infection in a juvenile orangutan (Pongo pygmaeus pygmaeus). J. Zoo. Wildl. Med. 2005;36(1):131-4.
7. Mansfield K, King N. Viral diseases. In: Bennett BT, Abee CR, Henrickson R, eds. Nonhuman Primates in Biomedical Research, Diseases. San Diego, CA: Academic Press; 1998:5-12.
8. Mätz-Rensing K, Jentsch KD, Rensing S, et al. Fatal Herpes simplex infection in a group of common marmosets (Callithrix jacchus). Vet Pathol. 2003;40(4): 405-411.
9. McGeoch DJ, Dolan A, Ralph AC. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 2000;74(22):10401-1046.
10. Rootman DS, Haruta Y, Hill JM. Reactivation of HSV-1 in primates by transcorneal iontophoresis of adrenergic agents. Invest Ophthalmol. Vis. Sci. 1990;31 (3):597-600.
11. Schäfer A, Lengenfelder D, Grillhösl C, et al. The latency-associated nuclear antigen homolog of herpesvirus saimiri inhibits lytic virus replication. J. Virol. 2003;77(10):5911-5925.