Signalment:
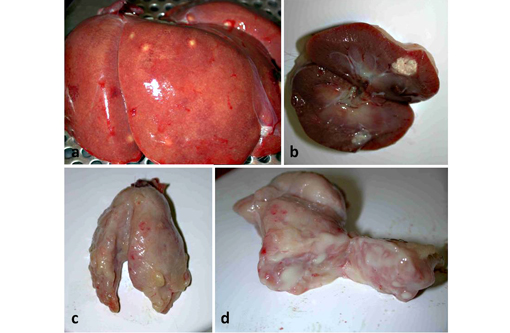
Gross Description:
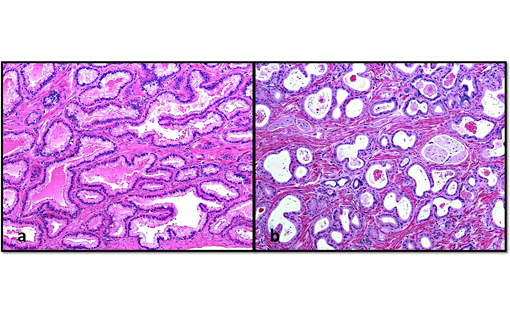
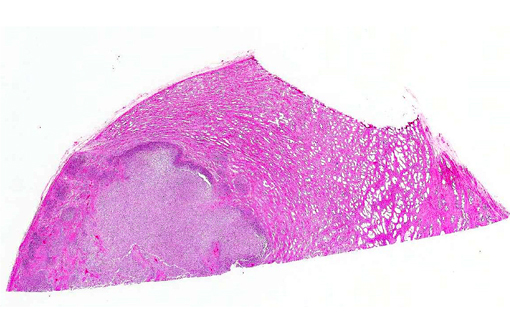
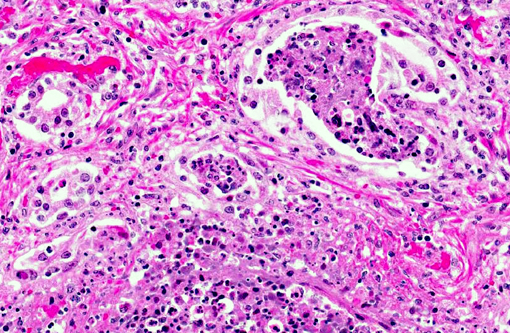
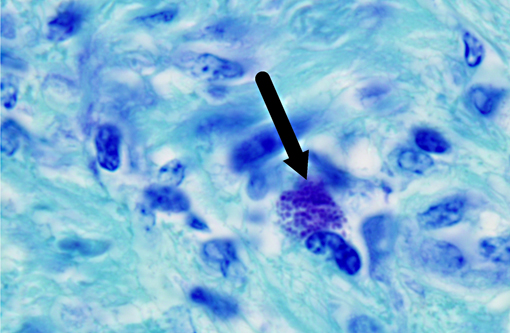
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
Burkholderia pseudomallei is a facultative anaerobic, saprophytic, gram-negative bacterium that causes melioidosis, a zoonotic multisystemic disease. The bacterium is rod-shaped with rounded ends and is often described as having a safety pin appearance.(4) It can survive hostile environmental conditions including acidic environments, wide temperature ranges, nutrient deficiencies and dehydration.(3) The disease is spread through inhalation, contamination of skin wounds, or ingestion from the environment. Direct transmission from infected to na+�-�ve animals and vertical transmission are rare.(9) It is designated as a Tier 1 select agent (requiring biosafety level 3 containment) by the US Centers for Disease Control due to its natural resistance to antibiotics, potential for easy dissemination and high mortality in humans and animals.(8,9) Sporadic cases of melioidosis have been reported in Central and South America but the disease is rare in North America.(9,12) As it is endemic in Southeast Asia, northern Australia and the Indian subcontinent,(9,12) melioidosis should be a differential diagnosis for nonhuman primates imported from Asia which develop abscesses or nonspecific signs of infectious disease, regardless of the time since importation.(9) The animal presented here had been considered healthy for 1 year before presentation.Â
Burkholderia pseudomallei is an opportunistic pathogen, affecting many mammalian and non-mammalian species. The clinical signs and lesions of melioidosis are variable, and while septicemia is common, multisystemic suppurative or caseous inflammatory lesions are also characteristic.(9) The disease is best described in ruminants and swine where it may result in subclinical to disseminated fatal disease, depending on the route of infection, infectious dose, strain virulence, and host immune status.(12) In nonhuman primates, including rhesus, stumptail and pigtail macaques, chimpanzees and orangutans,(9,10) the most common clinical signs include anorexia, wasting, listlessness, intermittent cough, nasal discharge and mild respiratory disease that can result in bronchopneumonia, and generalized weakness. Multisystemic abscessation is common.(10) Nerve damage and necrotizing osteomyelitis have also been described.(9) In horses, donkeys and mules, a closely related bacterium, B. mallei, causes pyogranulomatous lymphangitis of the respiratory tract and skin,(14) commonly called glanders and farcy, respectively. It primarily occurs in Africa, Asia, the Middle East and South Africa, and human infections are often fatal if not treated.(2)
In nonhuman primates the prostate is divided into the cranial and caudal regions which are labeled according to their proximity to the urinary bladder and seminal vesicles,(7) and have distinct histological characteristics. In humans, the prostate is divided into 4 regions: the peripheral, central and transitional zones, and the anterior fibromuscular stroma.(4,6) Since proliferative lesions differ between the regions of the human prostate, for example, carcinomas occur more frequently in the peripheral zone,(4,6) it is important to differentiate between the lobes of the prostate. In macaques, the cranial and caudal lobes of the prostate are analogous to the central and peripheral zones of the human prostate, respectively.(6,7) In addition to these anatomical similarities, some nonhuman primates (macaques, orangutans, chimpanzees, gorillas) express the prostate specific antigen (PSA), a marker of prostatic health in humans, enabling value as natural models for human prostatic disease.(5,6,7)
JPC Diagnosis:
Conference Comment:
Given that B. pseudomallei is a zoonotic and potentially deadly disease as well as a potential bioterrorist threat, a significant amount of research is currently being conducted with regards to the pathogenesis, treatment and prevention of melioidosis. A recent study explored the role of toll-like receptors in the immune response and morbidity/mortality of the disease. Toll-like receptors are surface pattern recognition receptors expressed by various cells that recognize exogenous microbial products and signal the presence of infection to the host. The binding of pattern-associated molecular patterns (PAMPs) to TLRs initiates transmembrane signaling, generally utilizing the MyD88 protein, which leads to the activation of NF kappa β transcription factors, MAPK signaling, the expression of inflammatory cytokines and activation of the innate and adaptive immune systems.(1) TLR2 and TLR4, which bind bacterial lipoprotein and lipopolysaccharide (LPS) respectively, have previously been shown to regulate host innate immune responses in humans with B. pseudomallei.(13) TLR5, which binds flagellin, is defective in a small subset of people with a specific genetic defect resulting in ineffective signaling; carriers of this defect have recently been shown to have improved survival in melioidosis. This has led to the supposition that melioidosis may induce a TLR5-dependent innate immune response, with the release of IL-10, IL-8 and IL-6, among other cytokines, and that people with non-functional TLR5 have reduced sepsis and lower mortality due to an impairment of this inflammatory response.(13)
In closing, conference participants briefly discussed one possible differential diagnosis for melioidosis in a non-human primate: Klebsiella pneumonia, sometimes referred to as the shipping fever of monkeys, can also cause abscess in multiple organs; however, the bacteria are usually evident microscopically.Â
References:
2. Balder R, Lipski S, Lazarus JJ, et al. Identification of Burkholderia mallei and Burkholderia pseudomallei adhesions for human respiratory cells. BMC Microbiology. 2010;10:250. [accessed 11 June 2013].Â
3. Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 2005;18(2):383416.
4. Epstein, JI. The lower urinary tract and male genital system. In: KumarV, Abbas AK, Fausto N, eds. Robbins and Cotran Pathologic Basis of Diseases. 8th ed. Philadelphia, PA: Saunders Elsevier; 2010:971-1004.
5. Lewis RW, Kim JCS, Irani D, et al. The prostate of the nonhuman primate: normal anatomy and pathology. The Prostate. 1981;2:51-70.
6. McNeal JE. Anatomy of the prostate: a historical survey of divergent views. The Prostate. 1980;1:3-13.Â
7. Mubiru JN, Hubbard GB, Dick EJ Jr., et al. Nonhuman primates as models for studies of prostate specific antigen and prostatic disease. The Prostate. 2008;68:1546-1554.Â
8. Peacock SJ, Schweizer HP, Dance DAB, et al. Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei [online report]. Emerg Infect Dis [serial on the Internet]. 2008;14(7) [accessed: 11 June 2013] http://wwwnc.cdc.gov/eid/article/14/7/07-1501_article.htm
9. Ritter JM, Sanchez S, Jones TL, et al. Neurologic melioidosis in an imported Pigtail Macaque. Vet Pathol. Published online 10 April 2013 [accessed 11 June 2013].
10. Sprague LD, Neubauer H. Melioidosis in animals: a review on epizootiology, diagnosis and clinical presentation. J Vet Med B. 2004;50:305-320.
11. Vietri, NJ, Deshazer D. Melioidosis. In: Medical Aspects of Biological Warfare. United States Department of Defense: Walter Reed Army Medical Center Borden Institute; 2008:147-150.Â
12. Warawa JM. Evaluation of surrogate animal models of melioidosis. Front Microbiol. 2010;1:141.
13. West TE, Chantratita N, Chierakul W, et al. Impaired TLR5 functionality is associated with survival in melioidosis. J Immunol. 2013;190(7):3373-3379.
14. Zachary JF. Mechanisms of microbial infections. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease. 5th ed. St. Louis, MO: Elsevier; 2012:147-241.Â