Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 17
5 February, 2020
Dr. Emily Corbin
Training Officer
CASE III: 19N153 (JPC 4134352).
Signalment: 3-month-old, male, mixed breed, Ovis aries, sheep.
History: In a property with 108 ewes with their lambs with average age of 3 months, 13 lambs got sick. Signals were first noticed by the animals? caretaker on April 4, 2019, and included paresis, decubitus, nasal discharge, and cough. On April 6, 2019, 10 affected lambs had died, two of which were submitted to necropsy. The remaining three were referred to the Veterinary Teaching Hospital (VTH). Clinical examination showed rales on pulmonary auscultation, hyperemic ocular mucous membranes, and mucous nasal discharge, and the lambs were treated with Se/Vit E, flunixin meglumine and Ceftiofur. Two of them completely recovered and one died on the second day from the start of treatment. The results of the bloodwork performed at the VTH are presented in Table 1. Some of the lambs the remained at the property presented a subclinical increase in the muscle enzymes activity. In total, three lambs were necropsied, and presented similar gross and histological lesions.
Lambs were fed with 33 kg of ration manufactured on the property that included soybean hull, soybean meal, calcium carbonate and 63 kg of silage from Mombasa grass. Mombasa silage was being offered for the last 48 days. Previously sorghum silage has been provided to the lambs. The lambs were vaccinated and dewormed at 30, 60 and 90 days. The ewes were fed the ration at night ewes spent the day in Tifton pickets.
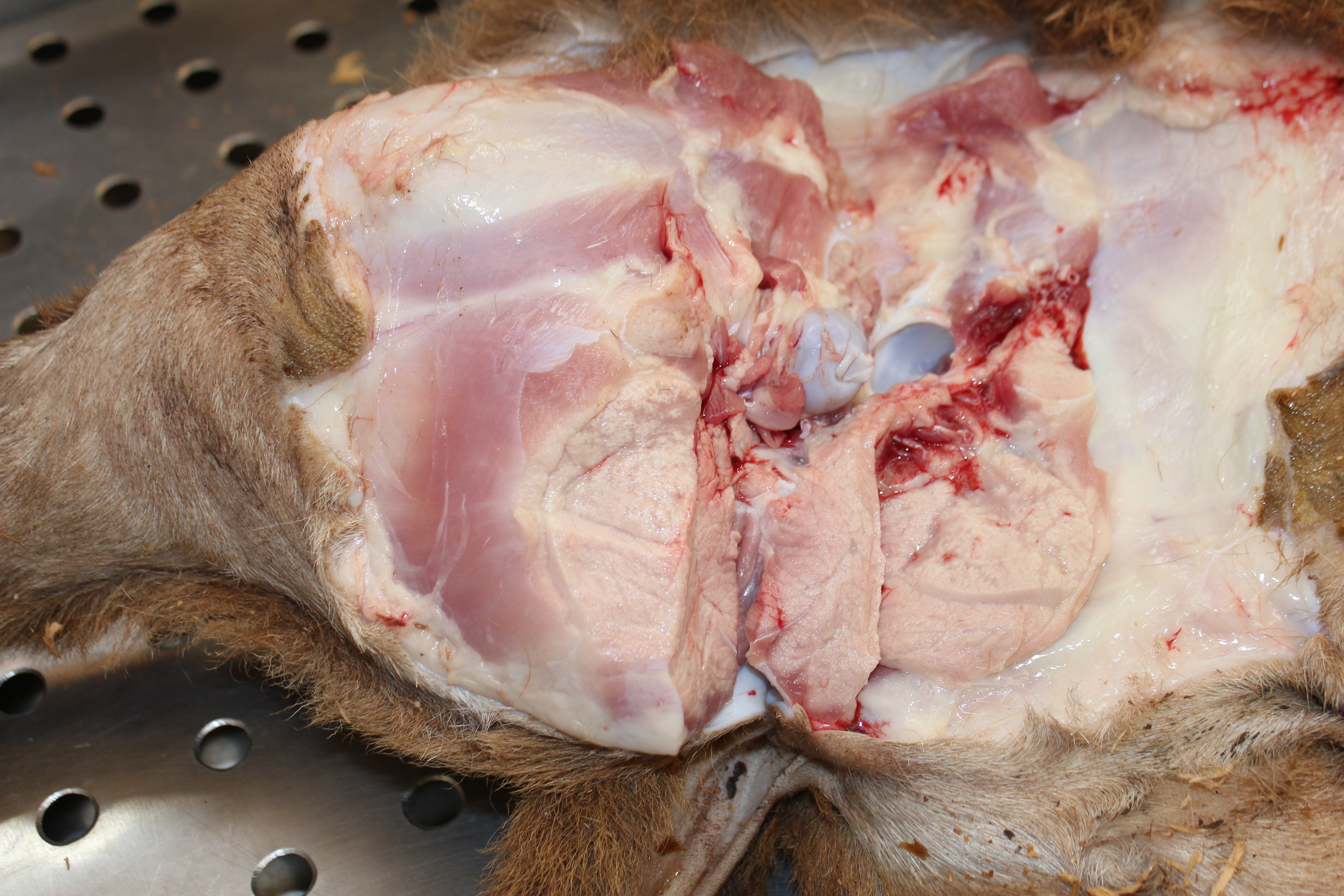
Gross Pathology: The cadaver is of an intact male lamb, undefined breed, with a brown wool coat with light areas, in a fair body nutritional status. The conjunctiva is of normal color, and the oral mucosa is pale. There is opacity of the left cornea (probably due to trauma while in decubitus). Multiple, focal, small to extensive, white-opaque chalk-like areas are observed in several muscle groups (Figure 1). The intensity of the lesions varied from mild (+) to severe (+++). These lesions distributed bilaterally and symmetrically in following muscles: supra-spinous (++), radial carpi (+), lateral digital extensors of the carpus, infra-spinosus (+), triceps (+++), brachiocephalic (++), gluteus medius (+++), semitendinosus (++), semimembranosus (+++), vastus medialis (+++), gastrocnemius (+++), vastus lateralis (++), longissimus dorsi (+++), diaphragm (++) (Figure 2), and tongue (+) and musculi colli.
The craniodorsal region of the right (the side of decubitus) lung is diffusely dark-red, firm and without crepitation. This aspect extends to portions the right caudal lobe. Large quantities of Haemonchus contortus were in the abomasal lumen, admixed with pasty dark-red abomasal content.
Laboratory results: See Table 1
|
Test |
♀1 |
♀2 |
♂3 |
Reference value |
|
RBC?s 106/µL |
10,89 |
13,59 |
7,3 |
9-15 |
|
Hemoglobin (g/dL) |
8,2 |
9,7 |
5,6 |
9-15 |
|
PCV (%) |
28,8 |
35,6 |
20,2 |
27-45 |
|
MCV (fL) |
26,4 |
26,2 |
27,7 |
28-40 |
|
MCH (g/dL) |
28,5 |
27,2 |
27,7 |
31-34 |
|
Leukocytes (mm3) |
25.500 |
14.600 |
7.600 |
4.000-12.000 |
|
Neutrophils (mm3) |
20.910 |
9.928 |
4.636 |
3.000-6.000 |
|
Lymphocytes (mm3) |
4.590 |
4676 |
1.000-9.000 |
|
|
Platelets (mm3) |
1.574.000 |
1.337.000 |
1.406.000 |
300.000-600.000 |
|
AST (UI/L) |
1.839,10 |
8.268,40 |
14.174,50 |
98-278 |
|
Creatinine (mg/dL) |
0,4 |
0,3 |
0,4 |
1,2-1,9 |
|
Creatine kinase (UI/L) |
2.068,10 |
14.333,70 |
57.384,50 |
8,1-12,9 |
|
GGT (UI/L) |
- |
- |
- |
20 a 52 |
|
BUN (mg/dL) |
- |
- |
- |
17,12 a 42,8 |
|
Tortal protein (g/dL) |
5,93 |
5,46 |
5 |
6,0 a 7,9 |
|
Globulin (g/dL) |
3,03 |
2,96 |
2,8 |
3,5 a 5,7 |
|
Albumin (g/dL) |
2,9 |
2,5 |
2,2 |
2,4 a 3,0 |
Table 1. Nutritional myopathy. Laboratory tests performed in the three affected lambs referred to the VTH. Lamb # 3 died. The other two recovered after treatment.
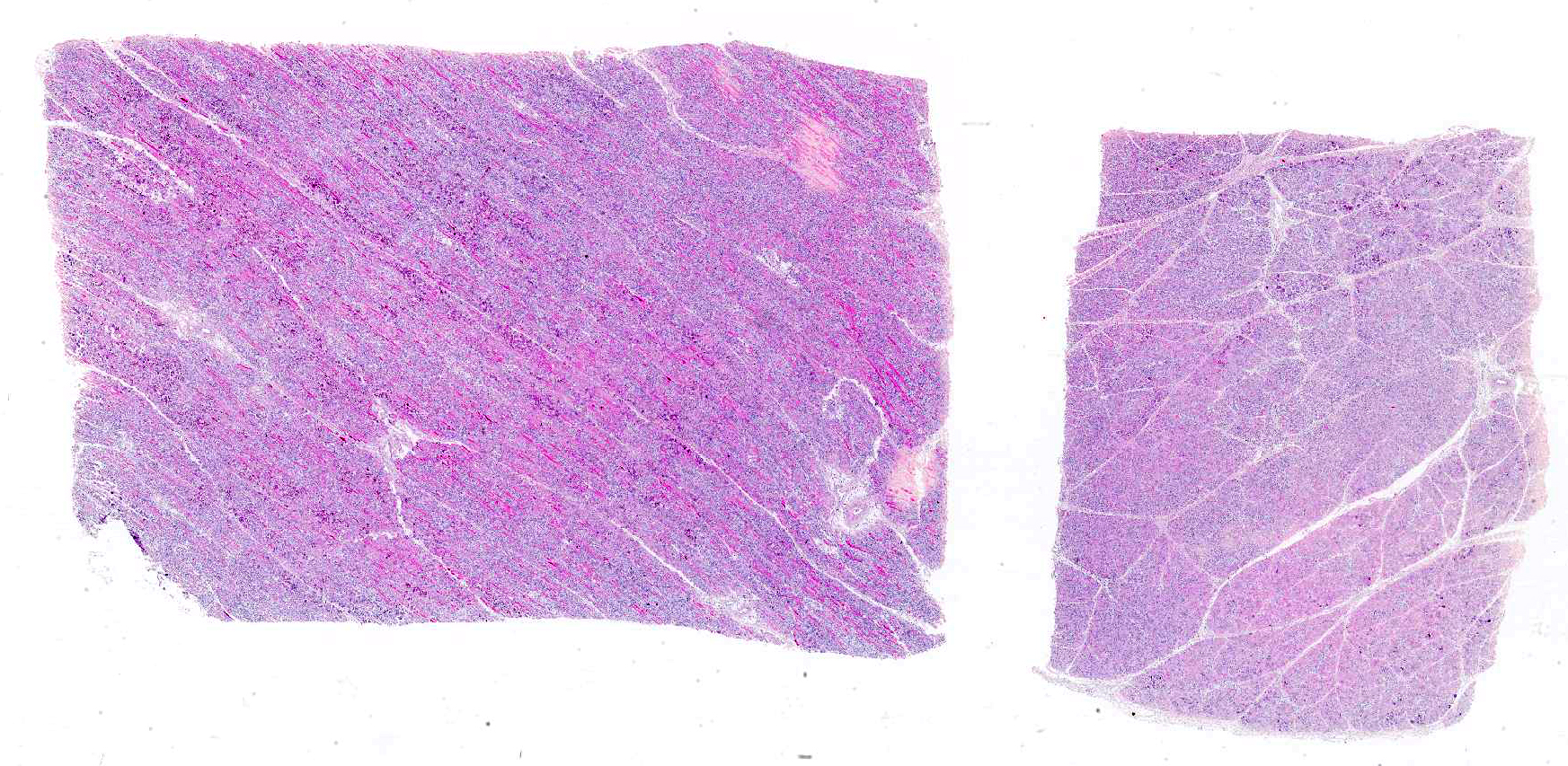
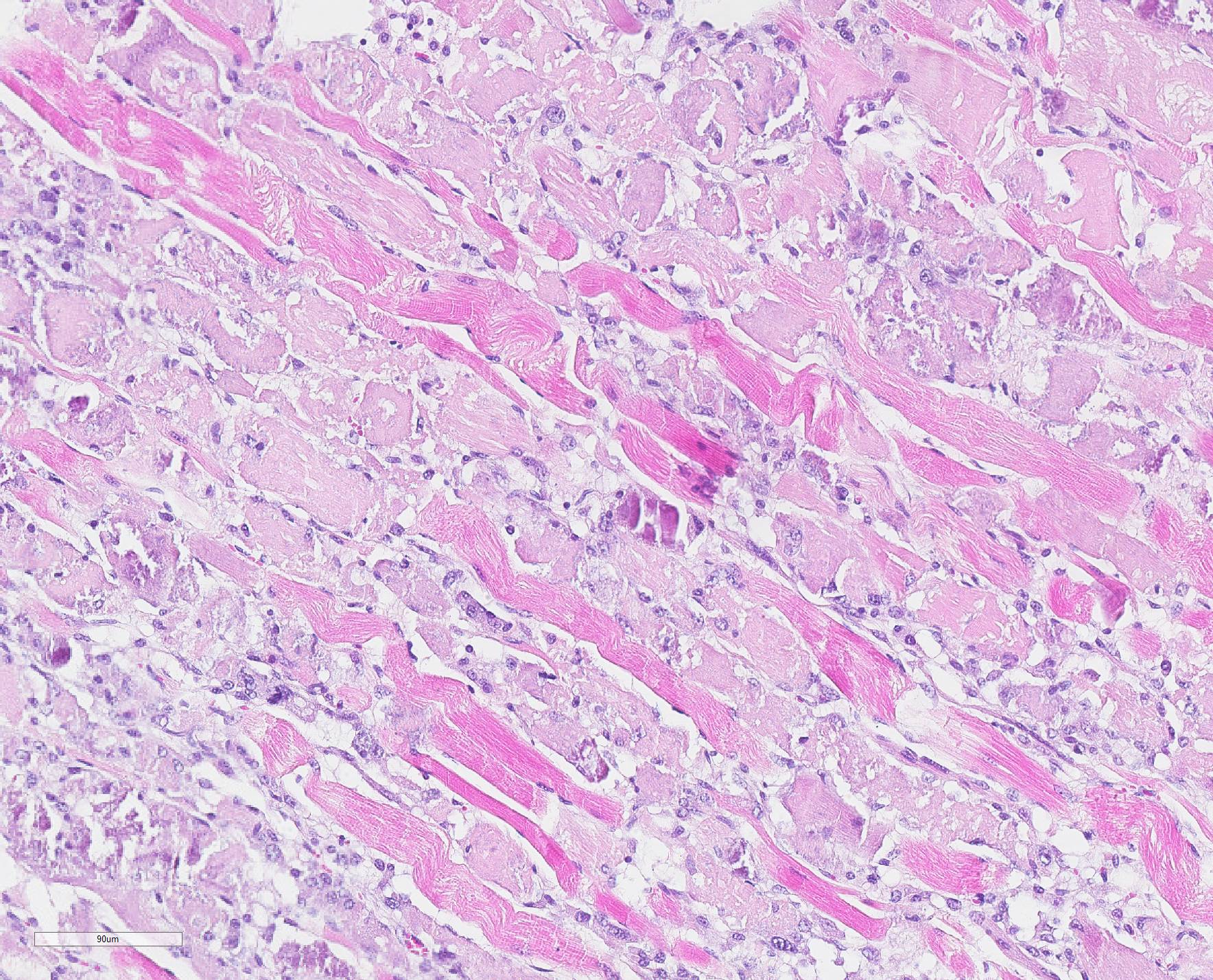
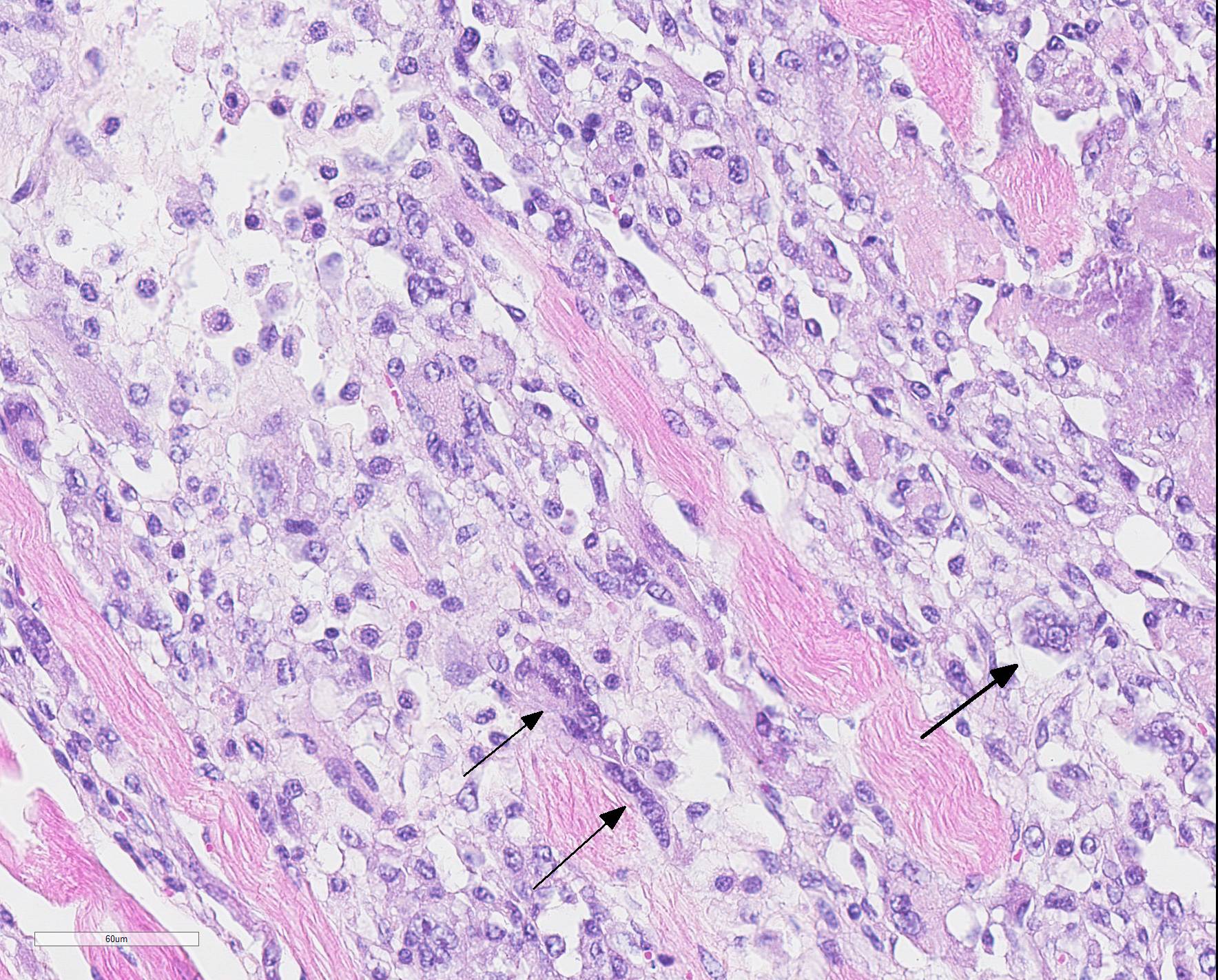
Microscopic Description: Skeletal muscle (semimembranous): Transverse and longitudinal sections of the skeletal muscles were analyzed. Multiple myofibers are either reduced in size or markedly swollen. Segments of affected myofibers are homogeneously hypereosinophilic, with loss of sarcoplasmic striations and, lumpy cleavage of many myofibers (floccular degeneration); the nucleus of these myofibers is pyknotic and shrunken; these changes are more evident in cross-sections. The sarcoplasm of the affected myofibers is moderate to markedly cluttered by fine to dense, basophilic, and birefringent granular material (mineralization). Randomly, in the endomysium, small foci of lymphocytes, plasma cells, and macrophages are seen. Also, satellite there is marked proliferation and swollen of satellite cells, and occasional multinucleated cells and nuclei with mitotic figures (attempts in regeneration) can be seen in the cytoplasm of degenerating/necrotizing myofibers. Some myofibers are slender, tapered and clear and contain multiple satellite cells arranged in rows in the sarcoplasm (myotubules). This latter finding is most often observed in longitudinal sections.
Within the lumen of pulmonary alveoli and bronchioles are small or moderate amounts of inflammatory cells, predominantly intact neutrophils. In the lumen of the alveoli, there is also amorphous eosinophilic material (edema), eosinophilic fibrillary material (fibrin) and moderate quantities of alveolar macrophages. Occasionally, in the bronchial lumen, small, long, slender fibers measuring about 100 μm in length and the slightly birefringent wall and light center are seen. Those structures are compatible with plant fibers. There are sparse hemorrhagic foci.
There is moderate hepatocellular vacuolar degeneration. There are no lesions in the spleen, kidney, adrenal, myocardium, intercostal, diaphragm, and encephalon.
Contributor Morphologic Diagnosis:
1) Necrotizing acute or subacute myopathy, multifocal, polyphasic, with prominent calcification and myofiber regeneration2) Lung, bronchopneumonia, suppurative, multifocal, moderate, associated with scattered intrabronchiolar vegetable fibers (consistent with aspiration pneumonia) (not submitted)
Contributor Comment Nutritional myopathies are important diseases of calves,1,18 lambs, swine,19 and foals,15 but has been described occasionally in more than 20 animals species including rabbits,13 birds,11,19 goats,17 reptiles,10 and man.19 The condition was first described in the 1930s and has since been attributed to Se/Vit E deficiency.13
The disease is a dominant part of the veterinary literature on muscle diseases8 and is also known as nutritional myodegeneration, white muscle disease, stiff-lamb disease, nutritional muscular myopathy (although use of this latter denomination is not recommended). Several syndromes are associated with deficiency of Se, Vit E, or both in domestic animals, such as nutritional myopathy, degenerative myelopathy in horses, degenerative encephalopathy in birds, retention of placenta in cattle, hepatosis dietetica in pigs, mulberry heart disease in pigs, exudative diathesis in pigs7 and chickens, steatitis (yellow fat disease) in cats, and immune system compromise;19 in people this nutritional deficiency is known in China for inducing a degenerative cardiomyopathy known as Keshan disease.19
The pathogenesis of nutritional myopathy due to Se/Vit E deficiency is not entirely understood. In these cases, as is customary, the usual suspects are lined up, and free radicals (FRs) are the main ones. Se/Vit E are responsible for the protection of cell membranes against the action of FRs, which mediate cell membrane damage.16 FRs are chemical specimens that have an unpaired electron in the most external orbit of the atom. FRs include reactive oxygen species such as superoxide (O2) and reactive nitrogen species such as NO.16 Because they possess this unpaired electron, FRs readily reacts with organic substances causing the peroxidation of the phospholipids, proteins and nucleic acids of the cellular membrane, leading the cell to necrosis. The protection of membranes is partly the responsibility of selenium-containing enzymes, and more than 30 selenoproteins are known7 and are part of the glutathione peroxidase/glutathione reductase system.4,9 If any condition deprives the organism from these mechanisms, the cellular membranes become physiologically defective, allowing the influx of calcium into the cytosol, resulting in the accumulation of calcium in the mitochondria. Damaged mitochondria fail to maintain the energetic needs of the cell, which results in cell necrosis, which manifests, in the myofiber, as floccular necrosis. The membrane injury allows the leakage of enzymes such as creatine kinase (CK), whose increased activity into the serum is a sign of muscle injury.7 One way to confirm the disease is by determining low levels of selenium in the food and carcasses of the affected animal, increased serum CK activity and histological findings of muscle degeneration.7
Several factors influence the transfer of Se from the soil to the plants, among them soil alkalinity (which favors the absorption of Se), type of plant (plants differ in their capacity to store selenium) and the presence of sulfur that competes with selenium, which is lower in the spring and in the rainy season.7,8
Data on selenium levels in Brazilian soils are scarce. However, outbreaks of nutritional myopathy are described in the South,2 the Northeast,1 and North3 of the country. In the South outbreaks occur mainly from July to August (winter, rainy season). Morbidity may be low but may reach 20%.
Feed with concentrates with a high content of fatty acids favors the occurrence of Se/Vit E deficiency. On the other hand, in animals fed concentrate, the availability of selenium may also be decreased as a consequence of the low pH of the rumen. Animals may die acutely without premonitory signs or after onset of apathy, dyspnea, and blood-filled foamy nasal discharge. There is marked tachycardia (150-200 bpm) and temperature is normal.7 In the acute form the treatment is usually ineffective. The most common form is the subacute with clinical course from a few days to a week, and mainly affects calves and lambs. The affected animals can be found in decubitus.
Clinical signs include muscle stiffness, difficulty in locomotion, muscle tremors, abnormal postures, apathy, and death. Occasionally it is possible to observe bilateral and symmetrical swelling of the gluteus, dorsolumbar muscles and muscles of the shoulders. The involvement of the diaphragm and the pharyngeal and esophageal muscles is responsible for the dysphagia seen in clinic cases.7 Dysphagia results in aspiration pneumonia, so typical in cases of nutritional myopathy2 and that occurred in the lambs of this outbreak. The subacute form (as in this report) generally responds well to treatment with Se/Vit E and animals recover within 3-5 days. Myoglobinuria is not a common sign in young animals but may occur sporadically in adult animals.8
Gross lesions are seen in the skeletal muscles and myocardium. They are bilaterally symmetrical and affected the muscles with a heavy load. The type of affected muscle varies with the age of the affected animal. In lambs the muscles of the neck and tongue (involved in suckling) are the most affected. In slightly older animals, muscles of the shoulder, thigh and diaphragm are most affected.8 Calcification of well-developed lesions leaves the chalky (opaque white) muscle, which gives rise to the name and ?white muscle disease.2
Microscopic changes in the myofibers vary somewhat between species, but are mostly the same. Usually they are polyphasic and include hyaline degeneration with loss of myofiber striations, floccular necrosis, marked proliferation of satellite cells, and an influx of neutrophils and macrophages into the sarcoplasmic tube. Secondary calcification is marked. The regeneration of muscle fibers is remarkable.13
The diagnosis is based on the characteristic clinical signs, associated to the clinical pathology and anatomopathological findings. Segmental necrosis of myofibers with calcification is typical of this disease, but not diagnostic. Confirmation of the diagnosis requires the determination of levels of selenium and α-tocopherol in tissues (renal cortex and liver for Se and liver for α-tocopherol). As the serum activity of glutathione peroxidase is highly correlated with blood levels of Se, the activity of this enzyme in the blood is used to assess levels of Se in tissues. Se and α-tocopherol analysis are useful because they differentiate cases of segmental myonecrosis from causes other than Se/Vit E deficiency.
The differential diagnosis should include toxic myopathies such as those caused by Senna occidentalis (coffee senna)5 and S. obtusifolia6 that frequently produce intoxication in cattle in this part the world, or by ionophore antibiotics, another common livestock toxicosis in this region. In cases of toxic myopathy, the agent must be investigated in the feed and the presence of the plant should be determined in the pasture. Some aspects of differential diagnosis are shown in Table 2.
|
Name of Condition |
Epidemiology and Pathology |
|
Senna occidentalis and S. obtusifolia toxicosis |
Degenerative lesions are marked in the skeletal muscles and very mild, if any, in the myocardium. Mineralization of muscle lesions are mild or absent. Mostly monophasic lesions. Myoglobinuria is a common clinical sign. Treatment with Se/vit E is ineffective.5 |
|
Ionophore toxicosis |
Ionophores are food additives, therefore the disease occurs in animals being fed commercial rations. Lesions are in both, myocardium and skeletal muscle. In ruminants, lesions are prominent in the myocardium. Mineralization of the lesions is not a feature. Mostly monophasic lesions. Myoglobinuria is part of the clinical picture. Treatment with Se/vit E is ineffective. |
|
Nutritional myopathy (Se/vit E responsive disease) |
Usually affects fast-growing young animals (2-4 month-old). Degenerative lesions occur in both skeletal and myocardial muscles. Myocardial lesions are not present in all cases.7 There is extensive mineralization of the lesions, which gives an opaque soft chalk-like gross appearance to the lesions (which does not occur in the other degenerative diseases mentioned in this table). Mostly polyphasic lesions. When instituted early in the course of disease treatment with Se/Vit E is effective. Myoglobinuria is usually not part of the clinical picture, except in sporadic cases in adult animals. |
|
Gossypol toxicosis |
Monogastric animals, such as pigs, are more susceptible to gossypol toxicity than ruminants. Necropsy lesions do not always include muscle degeneration (Mostly polyphasic lesions). Acute hydrothorax, hydropericardium, ascites and subcutaneous edema are part of necropsy findings. Hepatic lesions of centrilobular necrosis in pigs intoxicated with gossypol can be severe. |
|
Downer syndrome |
Usually affects well-nourished, well-fed, animals with large muscle masses. The lesion is well demarcated and focal or focally extensive and results from pressure (ischemia). Lesions tend to be polyphasic. It affects the parts in decubitus, usually those close to bony prominences. Muscle hemorrhages, ulcerative lesions on the skin that correspond to the decubitus site may be associated with these lesions and aid in the differential diagnosis. |
Table 2. Approaches to the differential diagnosis of some degenerative myopathies occurring in livestock in Brazil.
Contributing Institution:
Department of Pathobiology and Veterinary Science
Connecticut Veterinary Medical Diagnostic Laboratory
University of Connecticut
www.patho.uconn.edu
JPC Diagnosis: Skeletal muscle: Myocyte degeneration and necrosis, polyphasic, diffuse, moderate, with mineralization and rare myocyte regeneration.
JPC Comment: Another excellent and far-ranging discourse on the entity of nutritional myopathy by the contributor allows us to explore some less common avenues of Vitamin E deficiency, this time the human disease associated with hypovitaminosis E.
While there are eight different forms of Vitamn E, four each in the tocopherols and four in the tocotrienols (which all are absorbed in the small intestine), the liver can metabolize only one, alpha-tocopherol. The other seven forms are excreted. Low Vitamin E diets are seen in emerging countries; in developing countries, diseases impacting absorption are the most common cause. In the US, although studies have shown that 90% of men and 96% of women 19 years or older have diets deficient in Vitam E, only 0.1% of this demographic have deficient serum levels of alpha tocopherol.
The
most common causes of Vitamin E deficiency in humans include premature low
birth weight (premature infants have low vitamin E reserves due to limited
placental transfer), mutations in hepatic tocopherol transfer proteins, various
causes of fat malabsorption, cystic fibrosis (as patients are unable to secrete
pancreatic enzymes which help absorp within E, short bowel or inflammatory
syndromes, abetalipoproteinemia, and a genetic mutation of chromosome arm 8q.
The known beneficial effects of Vitam E in humans are in the areas of
antioxidants, immunomodulatory, and antiplatelet effects. Vitamin E
supplementations may impact the development of coronary artery disease by
preventing oxidative changes to LDLs. It enhances lymphocyte prolfieration
while decreased the production of prostaglandin E2 and serum lipid peroxides.
By the combination of decreasing LDL oxidation and reducing PGE2, this in turn
reduces platelet aggregation as well as inhibiting protein kinase C.
True disease is uncommon in hypovitaminosis E in humans. One of the manifestations arises from a lack of its antioxidant properties in the nervous system. One particular genetic disease arising from this deficiency is known as ataxia with isolated vitamin E deficency (AVED). This autosomal recessive disorder results from a mutation in the gene which codes for the alpha-tocopherol transfer portein and maps to chromosome 8q13. It manifests from the age 2 onward with ataxia, hyporeflexia, and difficulty with upward gaze. The deficient alpha-tocopherol transfer protein delivers Vitamin E to Purkinje cells and other neurons, leading to free redical stress, oxidative damage and cellular dysfunction in these cells. A similar condition, Friedreich ataxia, may mimic AVED, but results from a genetic mutation of the FXN gene, which results in oxidative stress due to iron buildup in neuronal mitochondria.
References:
1. Amorim SL, Oliveira ACP, Riet-Correa F, Simões SVD, Medeiros RMT, Clementino IJ. Distrofia muscular nutricional em ovinos na Paraíba. [Nutritional muscular dystrophy in sheep in Paraíba] Nutritional muscular dystrophy in sheep in Paraíba. Pesq Vet Bras. 2005;25(2):120-124. In portuguese.
2. Barros CSL, Barros SS, Santos MN, Metzdorf LL. Miopatia nutricional em bovinos no Rio Grande do Sul. Pesq Vet Bras. 1988;8:51-55. In portuguese.
3. Bezerra SFB, Batista JS, Melo DEB, Lima Neto, Souza E, Farias YJMD. Miopatia nutricional em ovinos no Rio Grande do Norte [Nutricional myopathy in sheep in Rio Grande do Norte] Acta Vet Bras. 2007;1(2):60-63. In portuguese.
4. Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB 1999; 13:1145-1155.
5. Carmo PMS, Irigoyen LF, Lucena RB, Fighera RA, Kommers GD, Barros CSL. Spontaneous coffee senna poisoning in cattle: report on 16 outbreaks. Pesq Vet Bras. 2011;31:139-146.
6. Carvalho AQ, Carvalho NM, Vieira GP, et al. Intoxicação espontânea por Senna obtusifolia em bovinos no Pantanal Sul-Mato-Grossense. [Spontaneous poisoning by Senna obtusifolia in cattle from the Pantanal of Mato Grosso do Sul] Pesq Vet Bras. 2014;34:147-152. In portuguese.
7. Constable PD, Hinchcliff KEW, Done SH, Grünberg W. Nutritional diseases and/or vitamin E deficiencies. In: Veterinary Medicine. A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 11th ed. Vol. 2. St. Louis, MO: Elsevier; 2017:1458-1478.
8. Cooper BJ, Valentine BA. Muscle and tendon: nutritional myopathy. In: Maxie, MG, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. 6th ed. Vol. 1. St. Louis, MO: Elsevier; 2016:214-218.
9. Da Costa LA, Badawi A, El-Sohemy A. Nutrigenetics and modulation of oxidative stress. Ann Nutr Metab. 2012;60(Suppl. 3):27-36.
10. Gabor LJ. Nutritional degenerative myopathy in a population of captive bred Uroplatus phantasticus (satanic leaf-tailed geckoes). J Vet Diagn Invest. 2005;17:71-73.
11. Giri DK, Miller DL, Thompson LJ, Mailler L, Styer E, Baldwin C. Superoxide dismutase expression and oxidative damage in a case of myopathy in brown pelicans (Pelecanus occidentalis). J Vet Diagn Invest. 2007;19:301-304.
12. Gordon N. Hereditary vitamin-E deficiency. Developmental Medicine & Child Neurology 2001; 43:133-135.
13. Hadlow WJ. Myodegeneration of nutritional origin. In: Innes JRM, Sauders LZ, eds. Comparative Neuropathology. New York, NY: Academic Press; 1962: 173-186.
14. Kennick TR, Coleman M. Vitamin E Deficiency. In: StatPearls, StatPearls Publishing LLC, Treasure Island, FL, 2019.
15. Löfstedt J. White muscle disease of foals. Vet Clin North Am Eq Pract. 1997;13:169-185.
16. Miller MA, Zachary JF. Mechanisms and morphology of cellular injury, adaptation and death: free radicals. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. Elsesvier, St Louis, MO: Elsevier; 2017:16.
17. Ross AD, Gee CG, Jackson ARB, Hall E, Greentree PL. Nutritional myopathy in goats. Aust Vet J. 1989;66(11):361-363.
18. Smith DL, Palmer SJ, Hulland TJ, McSherry BJ, Blackwell TE. A nutritional myopathy enzootic in a group of yearling beef cattle. Can Vet J. 1985;26:385-390.
19. Van Vleet JF, Ferrans VJ. Etiologic factors and pathologic alterations in selenium-vitamin E deficiency and excess in animals and humans. Biol Trace Elem Res. 1992;33:1-21.