Wednesday Slide Conference, Conference 3, Case 2
Signalment:
Adult, male, Göttingen minipig, Sus scrofa domesticus
History:
This source animal had an acute history of decreased activity, decreased appetite, and generalized orange-pink skin discoloration prior to initial test article administration. It was subsequently discovered laterally recumbent with an intermittent, abnormal breathing pattern. Bloodwork was collected immediately prior to euthanasia.
Gross Pathology:
There were no significant gross lesions.
Microscopic Description:
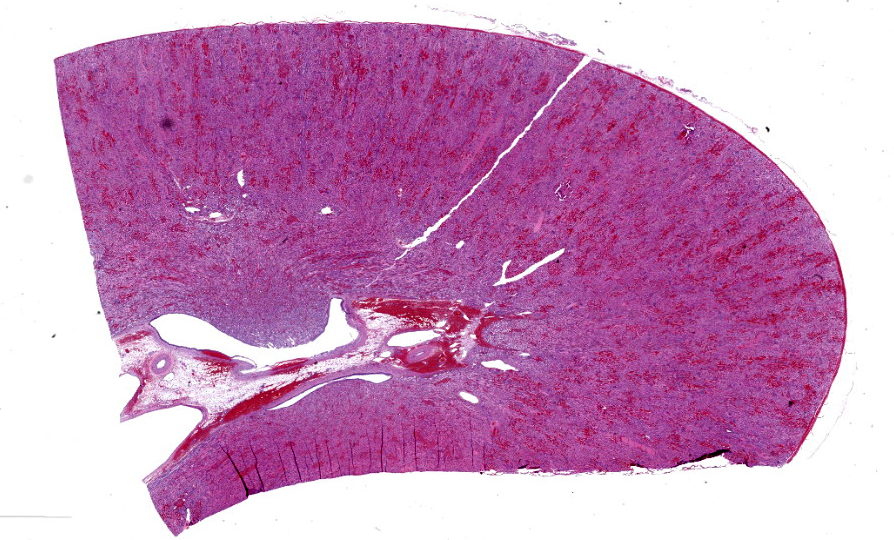
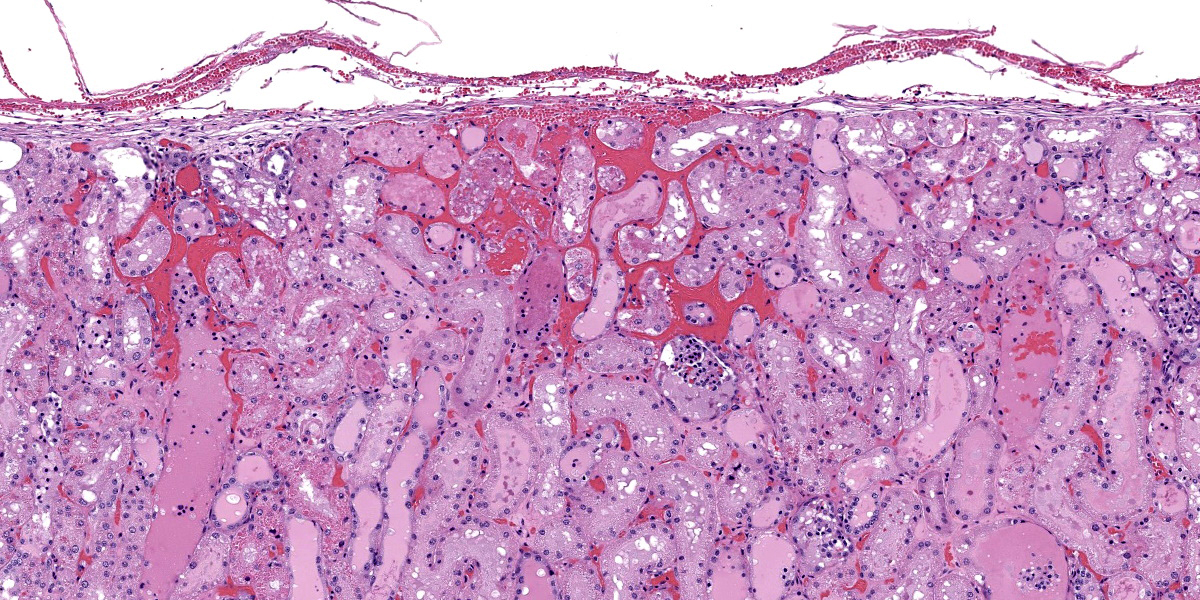
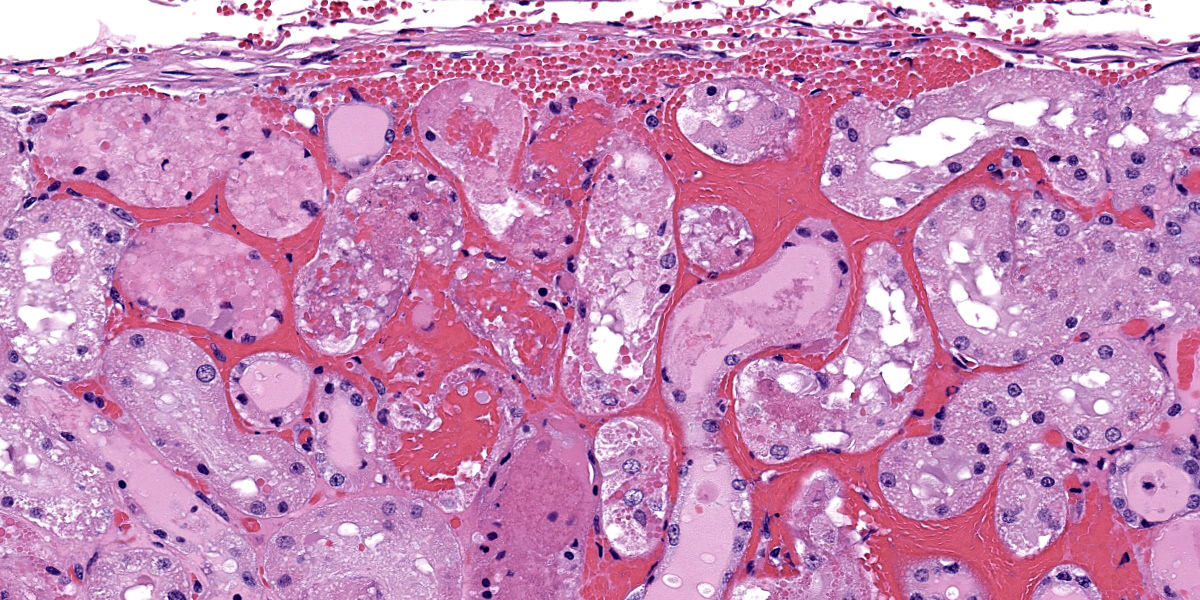
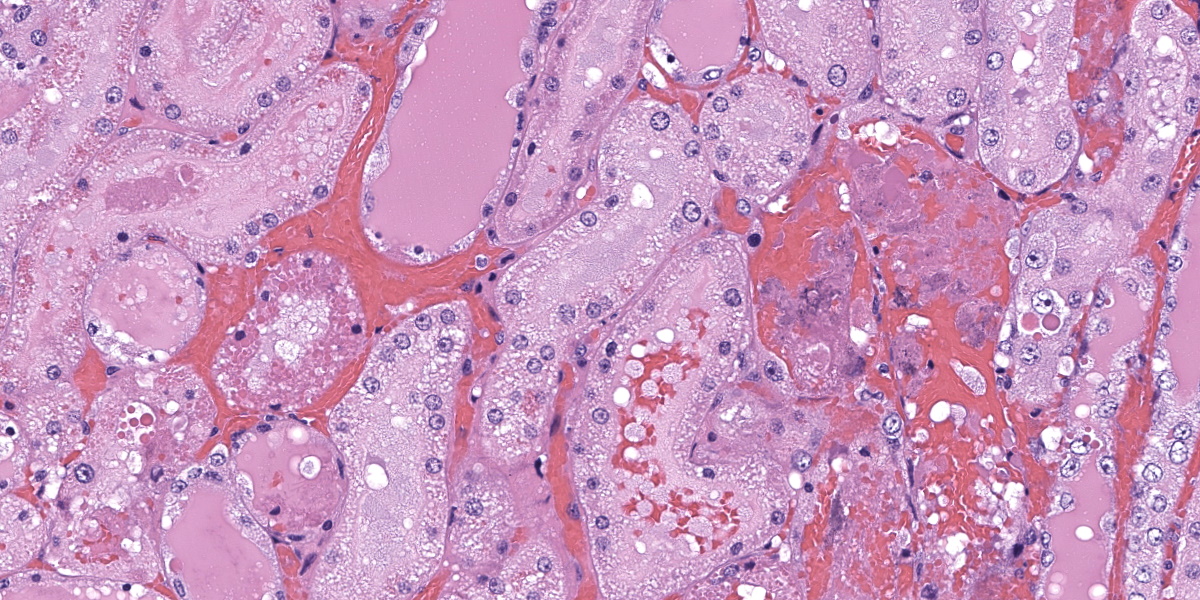
In the kidneys, renal tubules in the cortex and extending into the medulla are diffusely, mildly to moderately dilated and filled with eosinophilic proteinaceous fluid or erythrocytes. Epithelial cells lining effected tubules are multifocally flattened with basophilic cytoplasm and frequently contain hypereosinophilic, globular, cytoplasmic material. There are few scattered clusters of necrotic tubules within the cortex, characterized by hypereosinophilia, loss of differential staining, and nuclear pyknosis of the tubular epithelium. Occasionally, Bowman’s spaces are dilated and filled with similar eosinophilic fluid and hypereosinophilic globular material which moderately compresses glomerular tufts. There is moderate, multifocal expansion of the renal interstitium and peri-pelvic adipose tissue by abundant hemorrhage which surrounds and separates renal tubules and adipocytes.
Contributor’s Morphologic Diagnosis:
Kidney: Renal tubular hemorrhage and proteinosis, diffuse, acute, severe, with rare tubular degeneration and necrosis and diffuse interstitial hemorrhage
Contributor’s Comment:
Histologic lesions are compatible with hemorrhagic syndrome in Göttingen minipigs, also known as thrombocytopenic purpura syndrome (TP). Hemorrhagic syndrome has been previously described in Göttingen minipigs with thrombocytopenia as the underlying cause.1 Currently, the mechanism of the thrombocytopenia associated with hemorrhagic syndrome is unknown, but is thought to be secondary to an immune complex-associated disorder, specifically a type II-mediated thrombocytopenia and/or a type III-mediated vasculitis/glomerulonephritis.1,7 Animals between 7 weeks and 1 year have been reported as affected with no apparent hereditary etiology.1,6
Clinically, pigs suffering from hemorrhagic syndrome are typically anemic and severely thrombocytopenic (≤ 20,000/µl).1,6 Depending on the severity of the disease and the organs affected, additional bloodwork abnormalities such as increased liver or renal values may be present, as in this case. Macroscopically, widespread hemorrhage is a key feature in hemorrhagic syndrome, including petechial to ecchymotic hemorrhages or hematomas most commonly observed in the skin, heart, urinary bladder, and kidney.7 While there were no discrete hemorrhages observed macroscopically in this case, the generalized orange to pink skin discoloration and hyperbilirubinemia suggest that there was significant hemolysis in this animal.
Microscopically, lesions of hemorrhagic syndrome consist of multifocal interstitial to mucosal hemorrhages in multiple organs including but not limited to the skin, kidney, bladder, intestine, pancreas, lymph nodes, lungs, and skeletal musculature.1,7 In the kidneys, membranoproliferative glomerulonephritis is a common feature, characterized by thickening of glomerular basement membranes and an increase in the number of mesangial cells within glomerular tufts.1,6 Degenerative and proliferative vascular lesions affecting small to medium-sized muscular arteries and arterioles have also been reported in cases of hemorrhagic syndrome.6 Previously described vascular lesions range from endothelial cell hypertrophy and smooth muscle cell vacuolation to proliferation of the tunica intima, necrosis and thickening of the tunica media, and disruption of the internal elastic membrane.6 These lesions can also be accompanied by lymphohistiocytic to neutrophilic periarteritis.6 Vascular lesions are most commonly observed in the heart and renal pelvis, but can found in other affected tissues.6
Based on the rapid progression of clinical signs, lack of significant gross lesions and microscopic evidence of hemorrhage in the kidney, this case is considered to represent a peracute presentation of hemorrhagic syndrome in the Göttingen minipig. Certain histologic lesions typically observed in TP, such as hemorrhages in multiple organs and appreciable membranoproliferative changes in the kidney, were not present in this case, but may have developed later in the course of disease had this animal not been euthanized. The hypereosinophilic, globular material observed in the cytoplasm and lumen of renal tubules has been previously reported in cases of hemorrhagic syndrome and, in those cases, was confirmed to be IgG, IgM or C1q with immunohistochemistry, supporting an immune-mediated cause for the disease.1
Some differentials to consider for hemorrhagic syndrome in Göttingen minipigs include other hemorrhagic disorders such as neo-natal alloimmune thrombocytopenia and von Willebrand disease (VWD). Neonatal alloimmune thrombocytopenia occurs in piglets less than a week old and is associated with immune-mediated platelet destruction by alloantibodies produced in sow colostrum against fetal platelet antigens inherited from the boar.2 Von Willebrand disease is a dominant recessive, hereditary bleeding disorder caused by a mutation in von Willebrand factor.5 Type 3 VWD, the most severe form of the disease in which there is a near total absence of von Willebrand factor, has been previously reported in farm pigs and causes severe mucocutaneous and periarticular hemorrhage that can be fatal if not treated.5 While petechial to ecchymotic hemorrhages can occur virtually anywhere with hemorrhagic syndrome and may present similar to VWD grossly, VWD does not commonly cause visceral hemorrhages and has not been reported to cause membranoproliferative changes in the kidney.
Contributing Institution:
Charles River Laboratories, Mattawan, MI
www.crl.com
JPC Diagnosis:
Kidney: Hemorrhage, acute, multifocal to coalescing, marked with tubular degeneration, necrosis, regeneration, and proteinosis.
JPC Comment:
Case 2 ratchets up the pathology seasoning nicely, with our case discussion being particularly spicy. In this case, the widespread hemorrhage within the renal interstitium is an obvious feature, though the tubular changes should not be overlooked (Figures 2-2, 2-3, and 2-4). As this animal was euthanized and quickly necropsied, the cellular preservation of this section is excellent which negates the frustration of reading through autolytic changes. We agreed with the contributor that the hemorrhage noted fits with an acute interpretation of this lesion, though some participants quibbled that a true ‘peracute’ lesion would have no supporting histologic features as the animal would succumb too quickly. This however was the easy part of the case discussion.
Interestingly, a number of conference participants also raised the question of whether there could be underlying chronic changes in this case which we catalog in detail. Participants felt strongly that thrombocytopenic purpura syndrome would likely have some supporting chronic changes given the pathogenesis the contributor describes. We last saw this entity in Conference 3 in 2023-2024, though there are some key differences to note. In last year’s case, we reviewed multiple sections from the ear that featured arteriosclerosis with modest arteritis and periarteritis, including thrombi in section. In this case, there are some small- and medium-caliber blood vessels near the renal pelvis/hilus (figure 2-1) that have medial hypertrophy of the tunica media (‘onion-skinning’) with endothelial hypertrophy. Additionally, some participants noted rare foci of basophilic renal tubular cells that were stacked and had mitotic figures (interpreted as regeneration) which would support a chronic interpretation of this lesion. Within the collecting ducts, there are also multiple granular casts present which points towards a longer time course in this case as well. We looked carefully for ancillary changes that would align with this interpretation and ran both PAS and JMS (Jones Methenamine Silver) to highlight the glomerular basement membrane as well as a Movat’s pentachrome to examine the wall of blood vessels. We agree with the contributor that there were no glomerular changes in this case to include thickening of the basement membrane, discontinuity or deposits within the membrane, or any glomerular synechiae in any of the sections examined. Movat’s stain did show discontinuity of both the inner and outer elastic lamina in select vessels which was not readily apparent on H&E section. Nonetheless, we struggled to find a good example of vasculitis on our section as the lack of vascular change (vascular necrosis and/or surrounding inflammation) or obvious fibrin thrombi made it difficult to pin down the exact connection to the large degree of hemorrhage and subsequent tubular change in this animal. This could be related to thrombocytopenia, though we did wonder about the nature of the perimortem blood sample clotting and its relation to this case too. With these features in mind, we cautiously approached the morphological diagnosis for this case, and focused only on the features that we felt we had solid support for histologically. We thank the contributor for submitting this case as it prompted a fruitful discussion here at the JPC.
There are a number of potential rule outs for acute tubular injury and hemorrhage in swine. These include porcine circovirus (dermatitis and nephropathy syndrome), porcine respiratory and reproductive syndrome virus, classical swine fever, African swine fever, septicemia, and anticoagulant rodenticide ingestion.3 For Göttingen minipigs, background renal lesions are fairly limited and mild, and did not confound interpretation of this case.4
References:
- Carrasco L, et al. Immune complex-associated thrombocytopenic purpura syndrome in sexually mature Göttingen minipigs. J Comp Pathol. 2003 Jan;128(1):25-32.
- Forster LM. Neonatal alloimmune thrombocytopenia, purpura, and anemia in 6 neonatal piglets. Can Vet J. 2007 Aug;48(8):855-7.
- Imai DM, Cornish J, Nordhausen R, Ellis J, MacLachlan NJ. Renal tubular necrosis and interstitial hemorrhage ("turkey-egg kidney") in a circovirus-infected Yorkshire cross pig. J Vet Diagn Invest. 2006 Sep;18(5):496-9.
- Jeppesen G, Skydsgaard M. Spontaneous background pathology in Göttingen minipigs. Toxicol Pathol. 2015 Feb;43(2):257-66.
- Lehner S, et al. A 12.3-kb Duplication Within the VWF Gene in Pigs Affected by Von Willebrand Disease Type 3. G3 (Bethesda). 2018 Feb 2;8(2):577-585.
- Maratea KA, Snyder PW, Stevenson GW. Vascular lesions in nine Göttingen minipigs with thrombocytopenic purpura syndrome. Vet Pathol. 2006 Jul;43(4):447-54.
- Skydsgaard M, et al. International Harmonization of Nomenclature and Diagnostic Criteria (INHAND): Nonproliferative and Proliferative Lesions of the Minipig. Toxicol Pathol. 2021 Jan;49(1):110-228.