Wednesday Slide Conference, Conference 14, Case 2
Signalment:
Twin Oberhasli goat fetuses (kid A, kid B), Capra aegagrus.
History:
Received for necropsy examination in February were the bodies of twin Oberhasli goat fetuses (kid A, kid B) with placenta, reportedly stillborn/abortion. The history as stated by the submitter stated site of occurrence in San Pedro Valley River area of Arizona in the United states. Site on river, with a massive mosquito problem every year, also possible ticks; do treat to prevent but not usually effective. Unknown breeding date but doe was approximately 1-2 weeks from kidding based on ligaments starting to soften. Herd of 10, goats in good nutritional status. Put into fresh pasture 3 days prior to abortion with belly-high weeds, suspect mustard weed yellow flowers; pasture during day, hay at night, mineral supplement once weekly, dewormed twice annually, CDT immunization annually in March. Tested for Q fever, Caseous Lymphadenitis, Caprine Arthritis Encephalitis virus annually with specialty laboratory. These are the first kids born pre-term on farm in 20 years. Q fever reported in animals at another farm in area previously with one noted human testing positive from incident. Farm is a closed site, goats are inbred with only 1 buck, two generations inbred; buck is sire of dam and of preterm kids. Buck has malformed head. Fetal bodies may be bruised due to doe pawing aggressively at them.
Gross Pathology:
Examined are twin goat fetuses (kid A, kid B) and placenta, all of which are in good post-mortem condition. Kid A is a 4 lb. female with a 47 cm crown to rump length that is well-haired across the body surfaces. Kid A has mild vascular congestion of the meninges but is otherwise unremarkable on external evaluation. Kid B is a 4.5 lb. male with a 37.5 cm crown to rump length that is well-haired across the body. Kid B has prominent brachygnathism (craniofacial deformity). There is widespread, moderate edema of the body wall as well as a large amount of partially clotted blood free within the peritoneal cavity (maternal or partum induced trauma vs. tissue edema & hemoperitoneum). The placenta is 0.75 lb. and appears complete, with no grossly evident lesions; environmental debris is present on surfaces.
Laboratory Results:
Bunyavirus & Cache Valley Virus gel-based PCR, Liver/Lung/Placenta (Texas Veterinary Medical Diagnostic Laboratory): Cache Valley Virus detected.
Coxiella burnetii Q Fever rtPCR, Pooled Liver/Lung/Placenta (Texas Veterinary Medical Diagnostic Laboratory): Not detected.
Abortion Panel Livestock Bacterial Culture, Pooled Liver/Lung/Placenta (Texas Veterinary Medical Diagnostic Laboratory): Mixed bacterial growth, negative specialized culture for Brucella sp. and negative specialized culture for Campylobacter sp.
Trace Mineral Panel, Liver (Texas Veterinary Medical Diagnostic Laboratory): cobalt 0.16 ug/g (no reference range), copper 267.42 ug/g normal (normal: 100-600), iron 324.53 ug/g normal (normal: 200-520), manganese 14.54 ug/g normal (normal: 8.0-24.0), molybdenum 0.85 ug/g below normal (normal: >1.24 ug/g), selenium 2.32 ug/g normal (normal: 1.00-4.80), zinc 252.55 ug/g normal (normal: 100-480).
Microscopic Description:
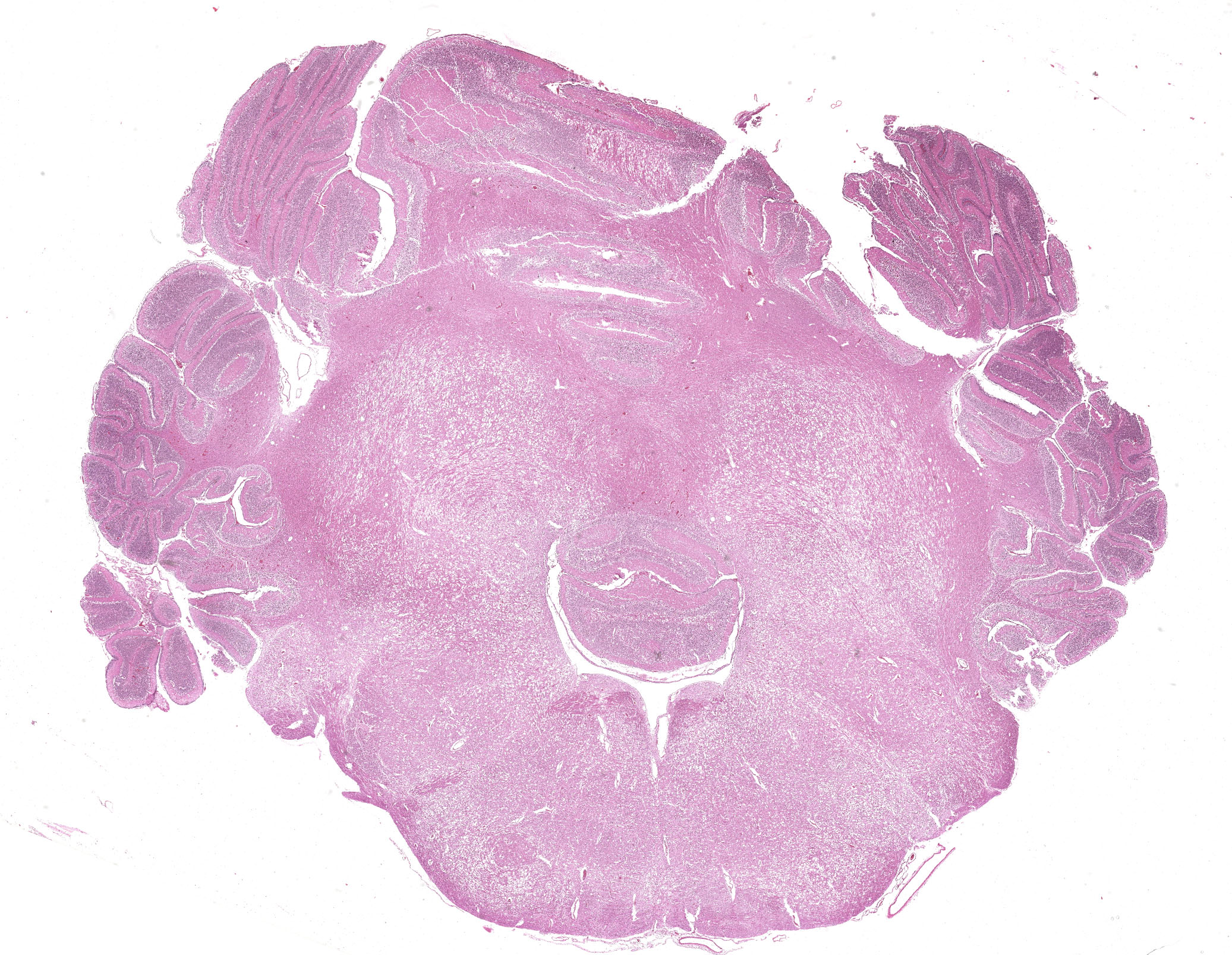
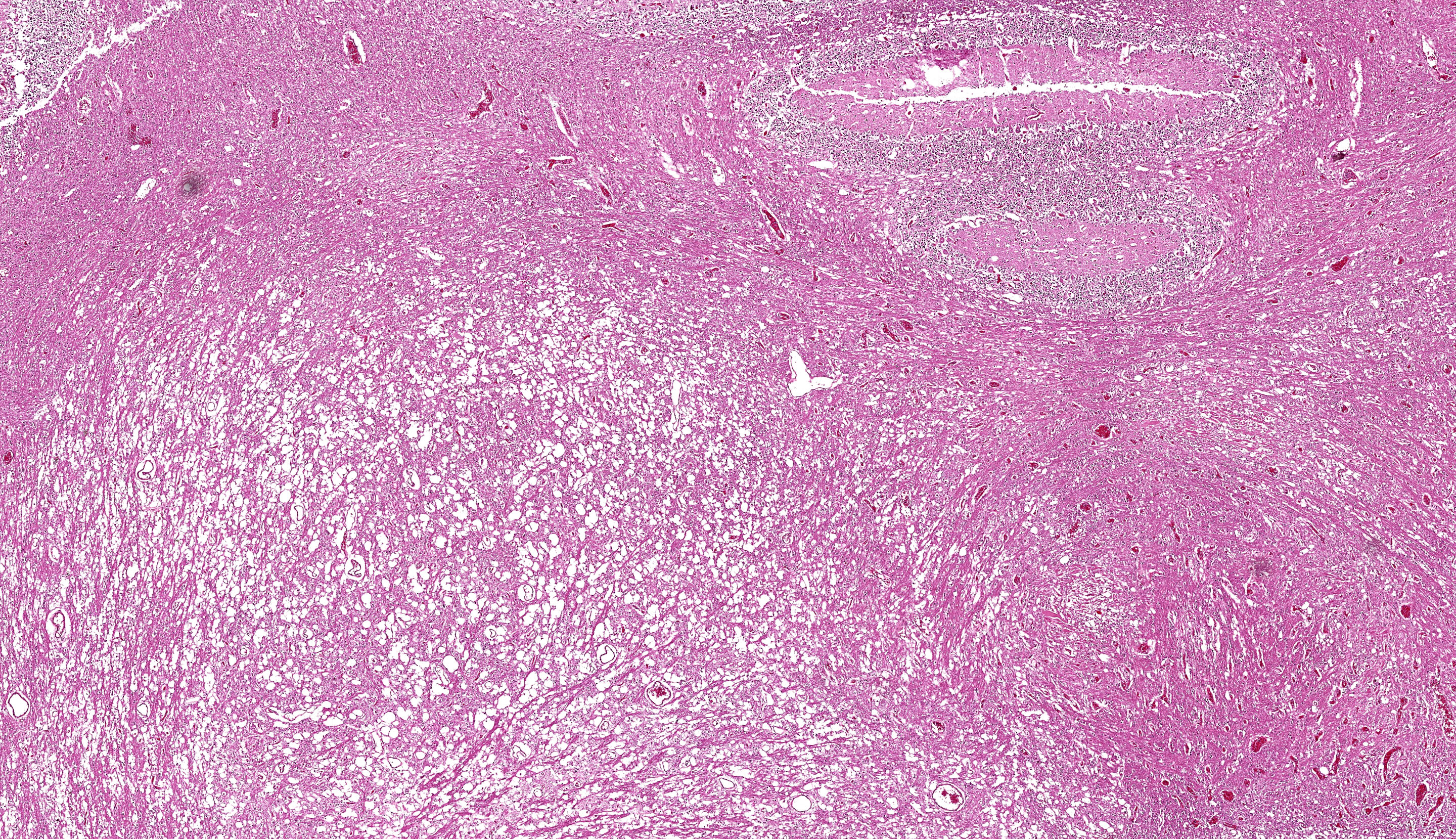
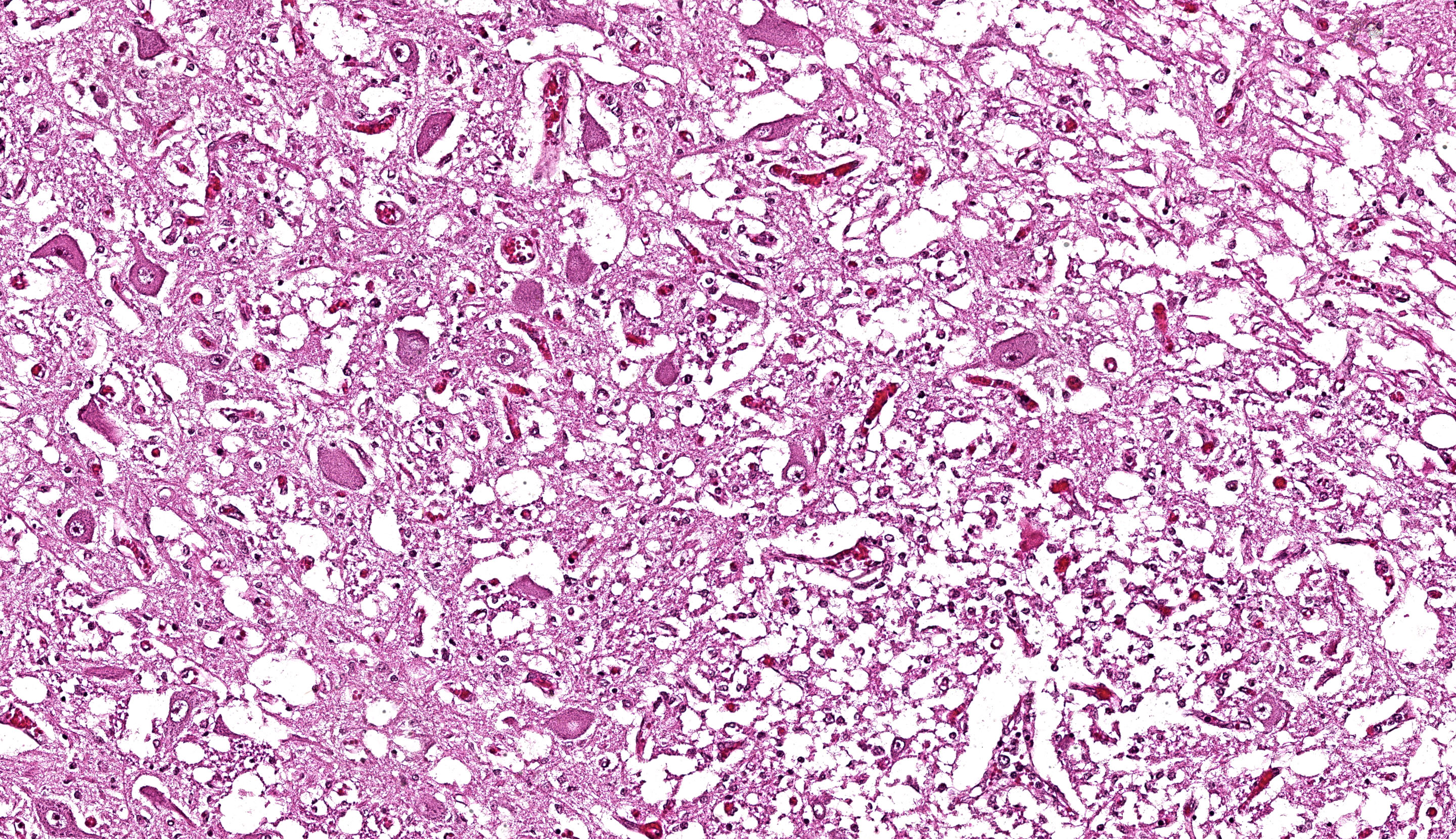
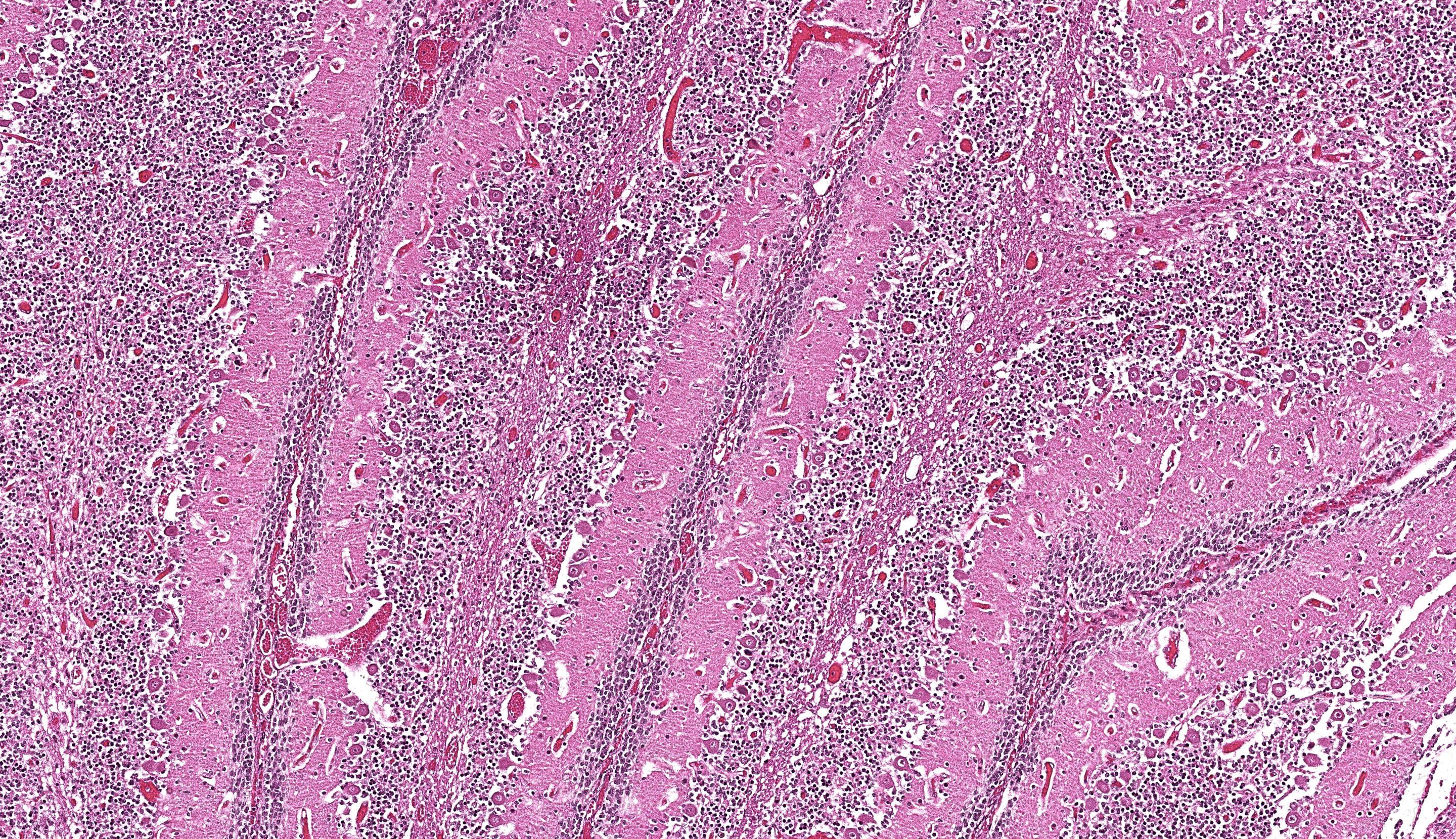
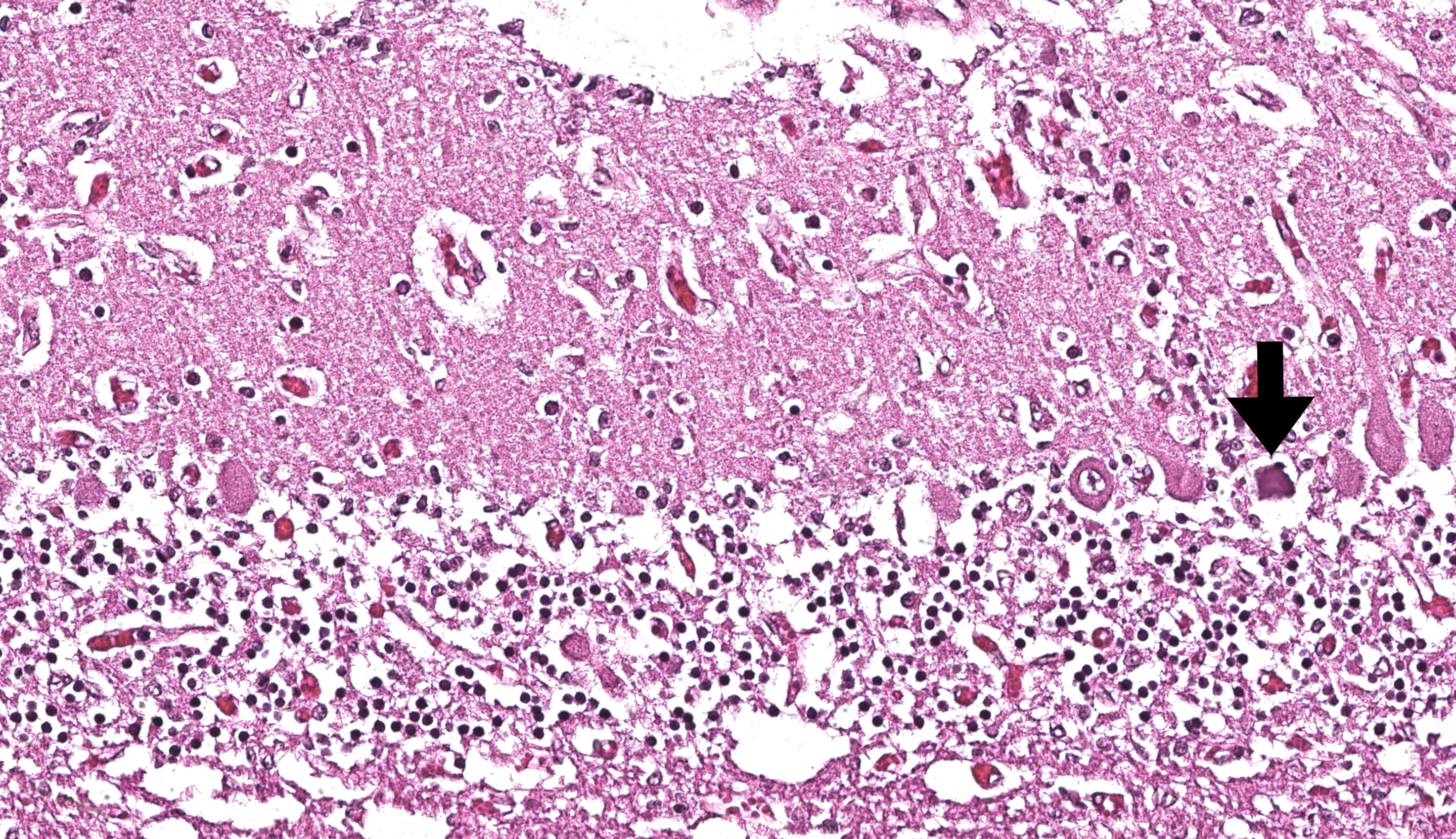
Brain: The brainstem, and to a lesser extent the cerebellum, has variably mild to marked rarefaction of the neuropil, expanded clear Virchow Robin spaces, white matter dilated axonal tracts rarely containing swollen axons, rare random small areas with loss of cellularity with small basophilic aggregates, and variably diminished to normal nuclear density within the granular layer of the cerebellum (kid B). The grey matter of the cerebrum appears hypercellular with areas of diminished or loss layering (kid A, kid B). There is mild to moderate vascular congestion throughout the brain (kid A, kid B).
Placental meconium staining noted (not represented on JPC WSC slide submission). No significant lesions noted on histologic evaluation of major organs from kid A, kid B.
Contributor’s Morphologic Diagnosis:
Brain: Rarefaction, hypoplasia, degeneration, locally extensive, grey and white matter, brainstem and cerebellum, marked (kid B)
Contributor’s Comment:
Gross, microscopic, and molecular diagnostics collectively confirmed Cache Valley virus in this case. Cache Valley virus (CVV) belongs to the viral family Bunyaviridae, genus Orthobunyaviridae, serogroup Bunyamwera.3 Cache Valley virus, Akabane virus, and Schmallenberg virus are each members of the genus Orthobunyaviridae. Orthobunyaviruses are usually maintained through arthropod-vertebrate-arthropod cycles.3 These viruses have a notable tropism for fetal tissues.3 In utero infection by these viruses causes prenatal losses and congenital deformities, including arthrogryposis and lesions in the brain ranging from hydrocephalus to hydranencephaly in certain mammalian hosts.3,4,6
Cache Valley virus is most frequently reported in sheep, but has been reported in other mammalian species.3,4 Cache Valley virus has been reported as a cause of embryonic losses, fetal malformations, abortions, and stillbirths in sheep and goats.1,3,4,6 Investigations on in utero infection of ovine fetuses show highest mortality with infection between 27-35 days gestation, highest incidence of congenital anomalies at 36-45 days gestation.6 Typical of orthobunyaviruses, Cache Valley virus is transmitted to mammals by infected carrier arthropod vectors, with the viral identification confirmed in certain mosquitoes as well as biting midges broadly across North America.3,4,6
Humans may potentially be infected if bitten by infected arthropod vectors, but direct mammal to mammal transmission has not been identified. Overall reported cases of Cache Valley virus causing clinical disease in humans is rare.7
Contributing Institution:
Arizona Veterinary Diagnostic Laboratory
https://azvdl.arizona.edu/
JPC Diagnosis:
Metencephalon: Neuronal necrosis and loss, multifocal, severe, with cerebellar hypoplasia
JPC Comment:
We thank the contributor for sharing this case with us. In particular, the long clinical history made for a good discussion of differentials and assessment of overall slide features. The group discussed ruminant abortion at length. Although Cache Valley virus was not among the group’s morphologic diagnoses based on review of the slide alone, other similar agents such as border disease (pestivirus) and caprine arthritis encephalitis (lentivirus) were popular picks.
We differed from the contributor in our morphologic diagnosis concerning the matter of myelination. The asymmetry in measured crown-rump length is interesting and suggests that one twin likely died several weeks before its sibling and the accompanying abortion. Dr. Brown weighed the potential lack of myelination within the developing brain of this fetus (i.e. myelination occurs in greater proportion later in development) as another possible interpretation of the rarefaction that is evident from subgross. Additionally, the lack of spheroids (axonal degeneration correlate) is suggestive that few mature axons had yet developed in this animal. Nonetheless, we were surprised at the lack of overt autolysis in the brain given the timeline we inferred.
We agreed with the contributor’s assessment of cerebellar hypoplasia in this case. From subgross, folia were small with thin layers and increased space between folia were suggestive of this decrease in size. The accompanying necrosis, degeneration, and loss of Purkinje neurons reinforced this interpretation. Subsequently, grey matter neurons were also affected.
The pathogenesis and early lesions of CVV have been previously described.5 Key gross findings included spinal deformity, arthrogryposis and oligohydramnios. Key microscopic findings in fetuses include necrosis in the central nervous system and skeletal muscle of 7 to 14 days postinfection, and hydrocephalus, micromyelia, and muscular loss after 21 to 28 days postinfection.5
The epidemiology of CVV remains incompletely understood, to include major transmission vectors and amplifying hosts across regions of North America.2 The role of climate change in distribution of CVV across wider geography is speculative, though CVV isolation from mosquito vectors is more common later in the season (late summer and early fall).2 Longer periods of warmer weather may also extend the breeding period for reservoir hosts and increase the number of naïve hosts to propagate the virus further.
References:
- Cantile C, Youssef S. Nervous System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th edition. Elsevier; 2016.
- Hughes HR, Kenney JL, Calvert AE. Cache Valley virus: an emerging arbovirus of public and veterinary health importance. J Med Entomol. 2023 Nov 14;60(6):1230-1241.
- MacLachlan NJ. Bunyaviridae. In: MacLachlan NJ, ed. Fenner’s Veterinary Virology. 5th edition. Elsevier; 2017.
- OIE Terrestrial Manual 2018. Chapter 3.9.1. Bunyaviral Diseases of Animals (excluding Rift Valley fever and Crimean-Congo haemorrhagic fever). Accessed June 1, 2021. https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.09.01_BUNYAVIRAL_DISEASES.pdf.
- Rodrigues Hoffmann A, Welsh CJ, Wilcox Varner P, de la Concha-Bermejillo A, Marchand Ball J, Ambrus A, Edwards JF. Identification of the target cells and sequence of infection during experimental infection of ovine fetuses with Cache Valley virus. J Virol. 2012 May;86(9):4793-800.
- Schlafer DH, Foster RA. Female Genital System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th edition. Elsevier; 2016.
- Centers for Disease Control and Prevention (CDC). Cache Valley Virus. Accessed June 1, 2021. https://www.cdc.gov/cache-valley/index.html