Signalment:
Gross Description:
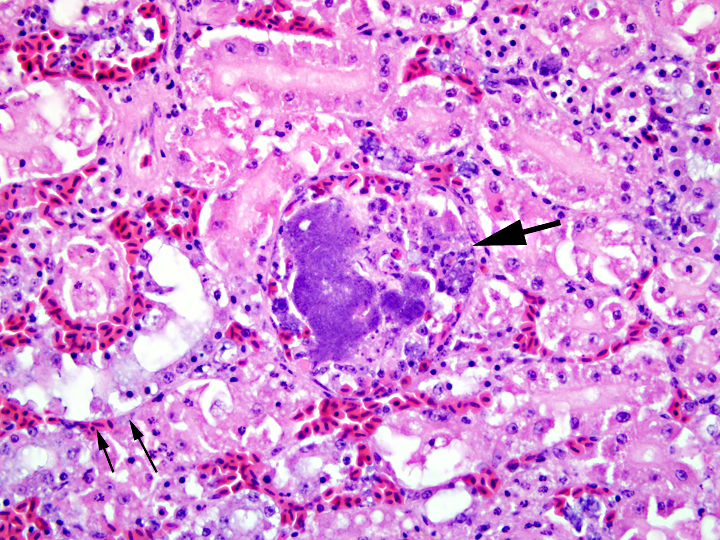
Histopathologic Description:
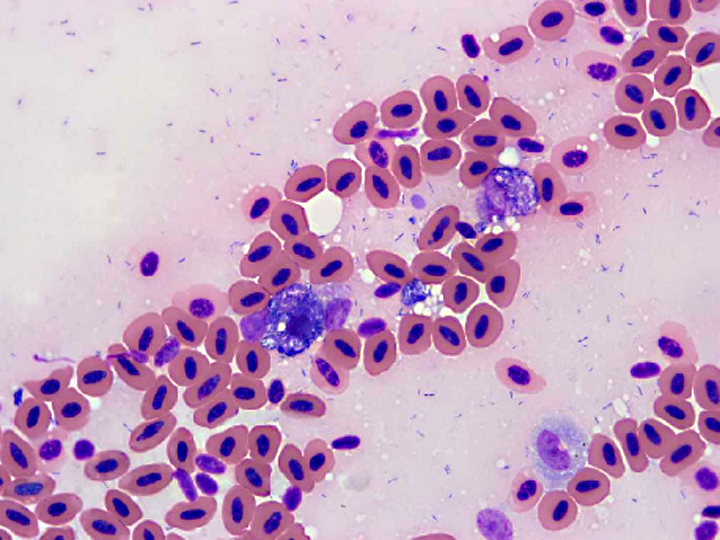
Other tissues: Large intracytoplasmic aggregates of bacteria similar to those in the kidney were present in phagocytic cells within vessels of most tissues. These were associated with hemorrhage and necrosis in the heart, spleen, and intestine. Histiocytosis was especially prominent in the spleen and was accompanied by a remarkably large number of intracellular bacteria. Multiple fibrinocellular thrombi were also present in the lung. Proliferative and ulcerative pododermatitis (bumblefoot) was confirmed and gram-positive and gram-negative rod bacteria, grampositive coccoid bacteria and superficial fungal hyphae were present in the ulcerated areas. Supplemental digital images include a composite of affected heart in which a vessel contains histiocytes with intracytoplasmic grampositive rod bacterial colonies.
Morphologic Diagnosis:
1. Kidney: intravascular histiocytosis with intracellular gram-positive rod bacteria
2. Kidney: mild to moderate multifocal acute tubular necrosis, mild multifocal granulocytic and lymphocytic interstitial nephritis, and moderate multifocal collecting duct ectasia with urate accumulation (not present in all sections)
Lab Results:
Condition:
Contributor Comment:
Infection of livestock with E. rhusiopathiaeresults in significant financial losses, particularly in swine and turkey production units. E. rhusiopathiae is considered normal pharyngeal flora in up to 50% of swine and bacteria also cause significant disease, most commonly septicemia, polyarthritis, and endocarditis.(8) The septicemic form in swine often leads to disseminated intravascular coagulation with classic cutaneous manifestations characterized by diamond-shaped pink to purple raised foci commonly referred to as diamond skin disease.(8) It causes a rapidly fatal septicemia in turkeys and other birds with inapparent to generalized petechiation in multiple organs and fibrinopurulent exudate on organ surfaces and joints.(1) In many cases there are limited histologic lesions, with colonies of intravascular bacteria with little or no inflammation as the primary feature.(3,11) While it may cause sporadic disease in a variety of wildlife, it is notable that E. rhusiopathiae has caused multiple significant mass mortality events involving hundreds of wild eared grebes in Nevada and brown pelicans in California, nearly an entire group of released captive raised kakapo in New Zealand, and one of 60 remaining Hawaiian -�-�Alala crows. (3,4,5,10)
Due to the ubiquitous nature of the bacteria, the potential for mass mortality events in birds, and the ability to infect individual birds of high value, E. rhusiopathiae should be considered as a potential cause of sudden death or sepsis in birds in zoological collections. The jacana in this case was wild caught in Tanzania and retained in the collection for approximately 12 years in multiple enclosures, most recently with a long-term mate, and had successfully raised several clutches of chicks. She had a history of proliferative pododermatitis lesions dating back to approximately 1 year after entering the collection. At necropsy the digital articular and skin lesions were chronic and proliferative, but also ulcerated and heavily colonized with opportunistic fungi and mixed bacteria. It hypothesized that infection in this case occurred via the skin wounds. An outbreak of E. rhusiopathiae affecting more than 300 chuckars was attributed to foot trauma caused by relocation from enclosures with solid flooring to wire-bottomed flooring.(2) Exposure to bedding material previously used by swine was the suspected source of the E. rhusiopathiae in the chukars. Disease in the jacana was suspected to have resulted from contamination of plantar skin wounds by bacteria in soil or enclosure bedding, water, mud, or other aquatic source associated with the stream. Bacteriologic surveys for potential sources were not evaluated in this case however, and dietary or other alternative sources including rodents or stray wildlife could not be ruled out.
JPC Diagnosis:
Conference Comment:
Erysipelothrix rhusiopathiae adheres to cells by producing neuraminidase, an enzyme that cleaves alpha glycosidic linkages in neuraminic acid, a reactive mucopolysaccharide in cell membranes. Erysipelothrix rhusiopathiae has hemagglutinating activity that can result in complement-dependent hemolysis in severe acute disease. The hemagglutinating activity is believed due to the high neuraminidase activity in virulent strains(6, 10).
Acute to subacute systemic erysipelothricosis causes generalized septicemia and endothelial swelling of capillaries and venules, leading to adherence of monocytes to vascular walls and thus vascular fibrinoid necrosis, fibrin thrombosis, and invasion of vascular endothelium by bacteria. Chronic erysipelothricosis leads to polyarthritis, synovitis, fibrosis and articular cartilage destruction. Vegetative valvular endocarditis frequently occurs, resulting in vasculitis, myocardial infarcts, destruction of valve endocardium, and splenic and renal infarcts(6, 10). Erysipelothrix rhusiopathiae is on a short list of gram positive bacilli important in veterinary medicine; others include Actinomyces spp., which are filamentous, Corynebacterium spp., and Listeria monocytogenes.
References:
2. Butcher G, Panigrahy B. An Outbreak of Erysipelas in Chukars. Avian Dis. 1985;29(3):843-845
3. Franson JC, Galbreath EJ, Wiemeyer SN, et al. Erysipelothrix rhusiopathiae Infection in a Captive Bald Eagle (Haliaeetus leucocephalus). J Zoo Wildl Med. 1994;25(3):446-448.
4. Gartrell BD, Alley MR, Mack H, et al. Erysipelas in the critically endangered kakapo (Strigops habroptilus). Avian Pathol. 2005;34(5):383-7.
5.Jensen WI, Cotter SE. An outbreak of erysipelas in eared grebes (Podiceps nigricollis). J Wildl Dis. Oct 1976;12(4):583-586.
6. Maxie MG, Robinson WF: Cardiovascular system. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 3, pp. 27-29. Academic Press, San Diego, California, 2007
7. Mazaheri A, Lierz M, Hafez HM: Investigations on the pathogenicity of Erysipelothrix rhusiopathiae in laying hens. Avian Diseases 49(4): 574-576, 2005
8. Qinning Wang Q, Chang B, Riley T. Review: Erysipelothrix rhusiopathiae Vet Micro. 2010;140: 405417.
9. Shibatani M, Suzuki T, Chujo M, Nakamura K. Disseminated intravascular coagulation in chickens inoculated with Erysipelothrix rhusiopathiae. J Comp Pathol. 1997;117(2):147-156.
10. Thompson K: Bones and joints. In: Jubb, Kennedy, and Palmer's Pathology of Domestic Animals, ed. Maxie MG, 5th ed., vol. 1, pp. 163-164. Academic Press, San Diego, California, 2007
11. Work TM, A Donna Ball, B and Mark Wolcott Erysipelas in a Free-ranging Hawaiian Crow (Corvus hawaiiensis) Avian Dis. 1999; 43:338-341.