Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 3
4 September, 2019
Dr. Corrie Brown, DVM, PhD, Diplomate ACVP
Josiah
Meigs Distinguished Teaching Professor
University Professor
Department
of Pathology
University of Georgia College of Veterinary Medicine
2200 College Station Road Athens, GA, 30602
CASE II: 14-102 (JPC 4048789).
Signalment: 8 month old purpose bred Dorset cross ewe (Ovis aries)
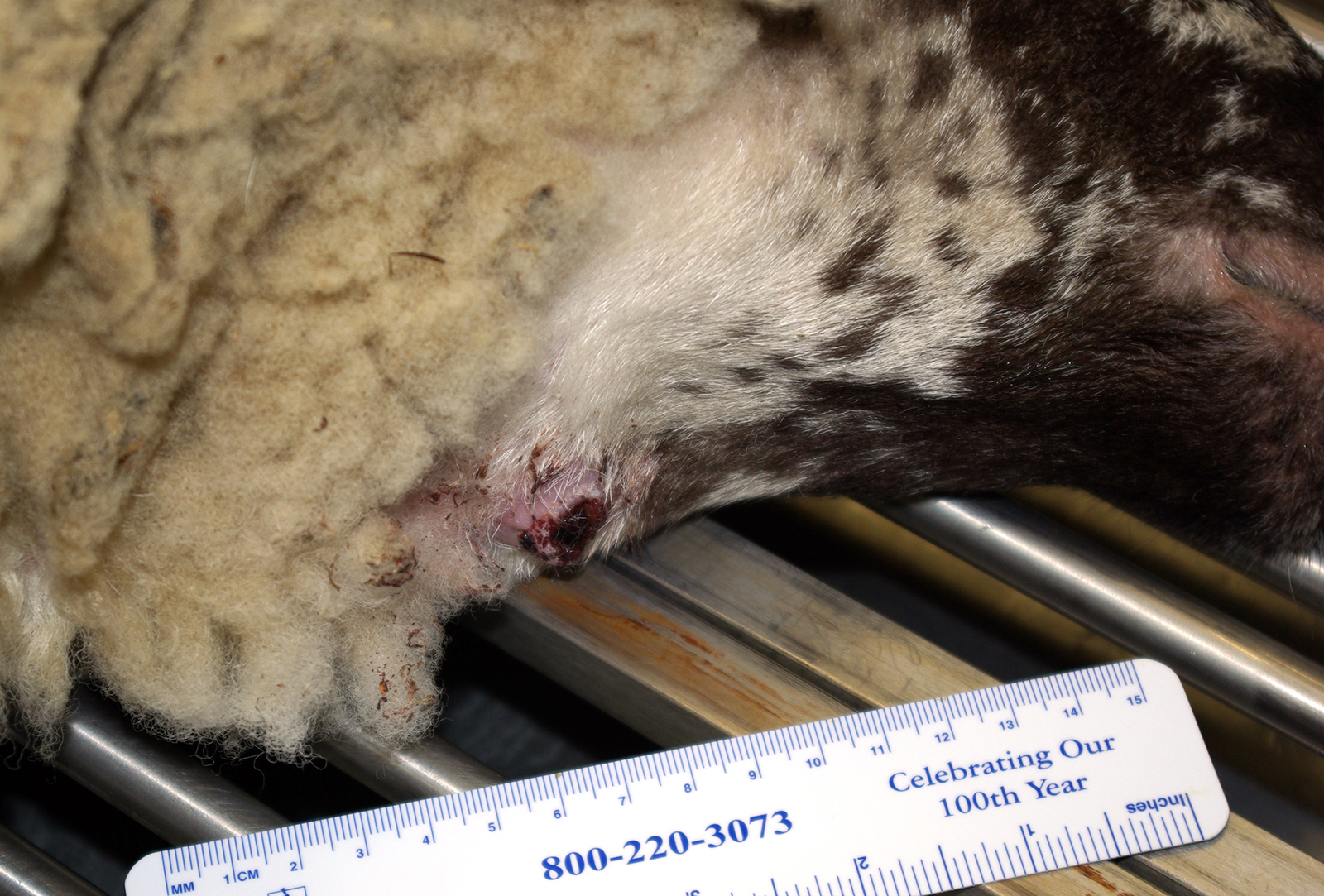
History: Received from supplier 17 days prior to euthanasia. Vaccinated for CD&T, pasteurellosis and orf 6 months prior. Negative Q fever serology. Dewormed with an avermectin at time of shipping. Eleven days after arrival she developed a 5 cm diameter subcutaneous abscess of the cranioventral neck near the angle of the mandible. FNA was performed and a sample was submitted for culture. The next day the abscess had ruptured and incompletely drained. When culture results were received, the decision was made to euthanatize.
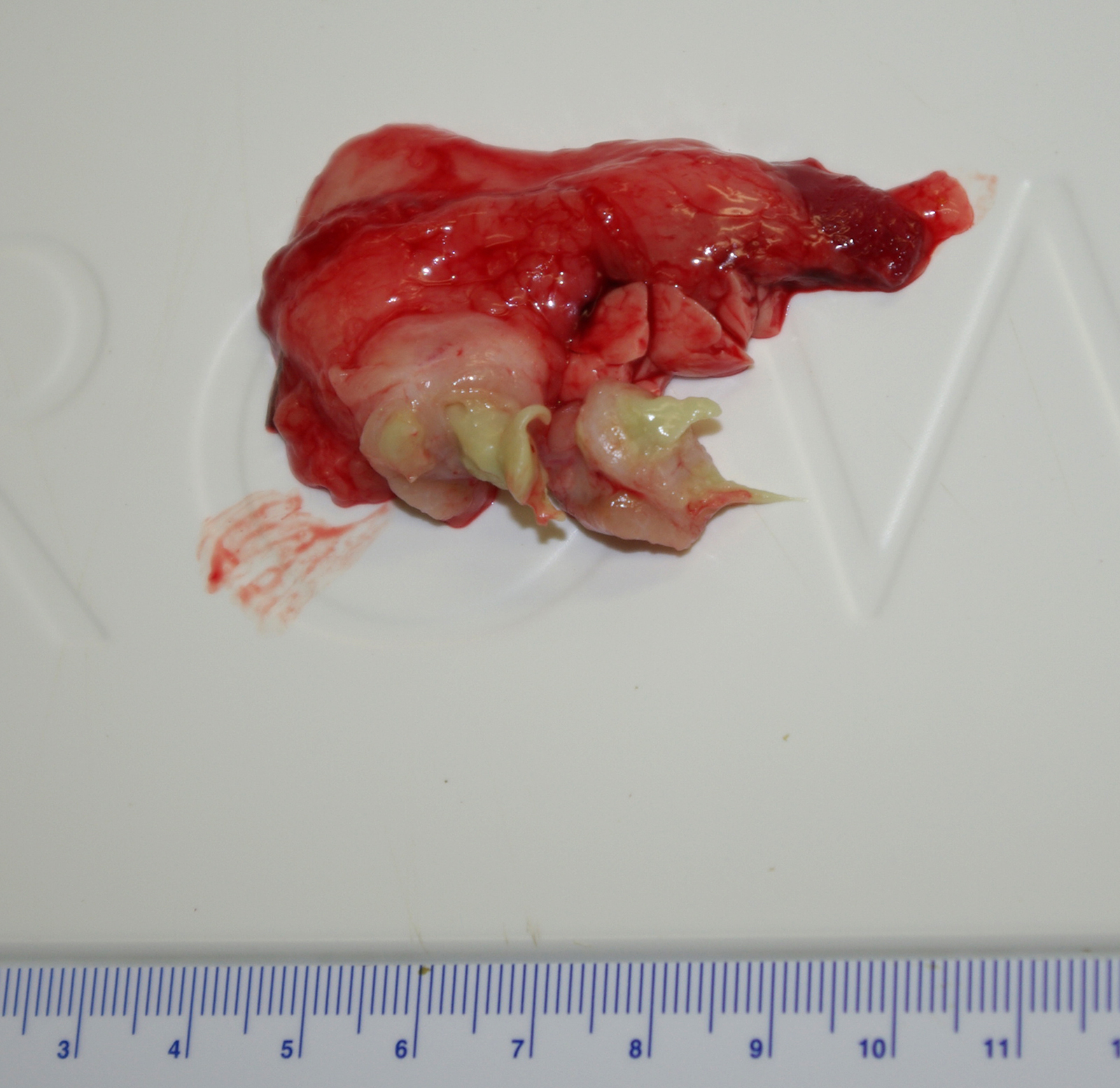
Gross Pathology: At necropsy there was a firm fibrous subcutaneous swelling at the angle of the right mandible with a cutaneous scab. The left submandibular lymph node was firm (fibrosis) and exuded thick green material on cut section. There were several small (2-5 mm) tan nodules in the lungs and liver.
Laboratory results: Gram Stain of FNA: Large numbers of Gram positive rods.
Aerobic culture of abscess (PADLS PVL): Heavy growth of Corynebacterium pseudotuberculosis.
Microscopic Description:
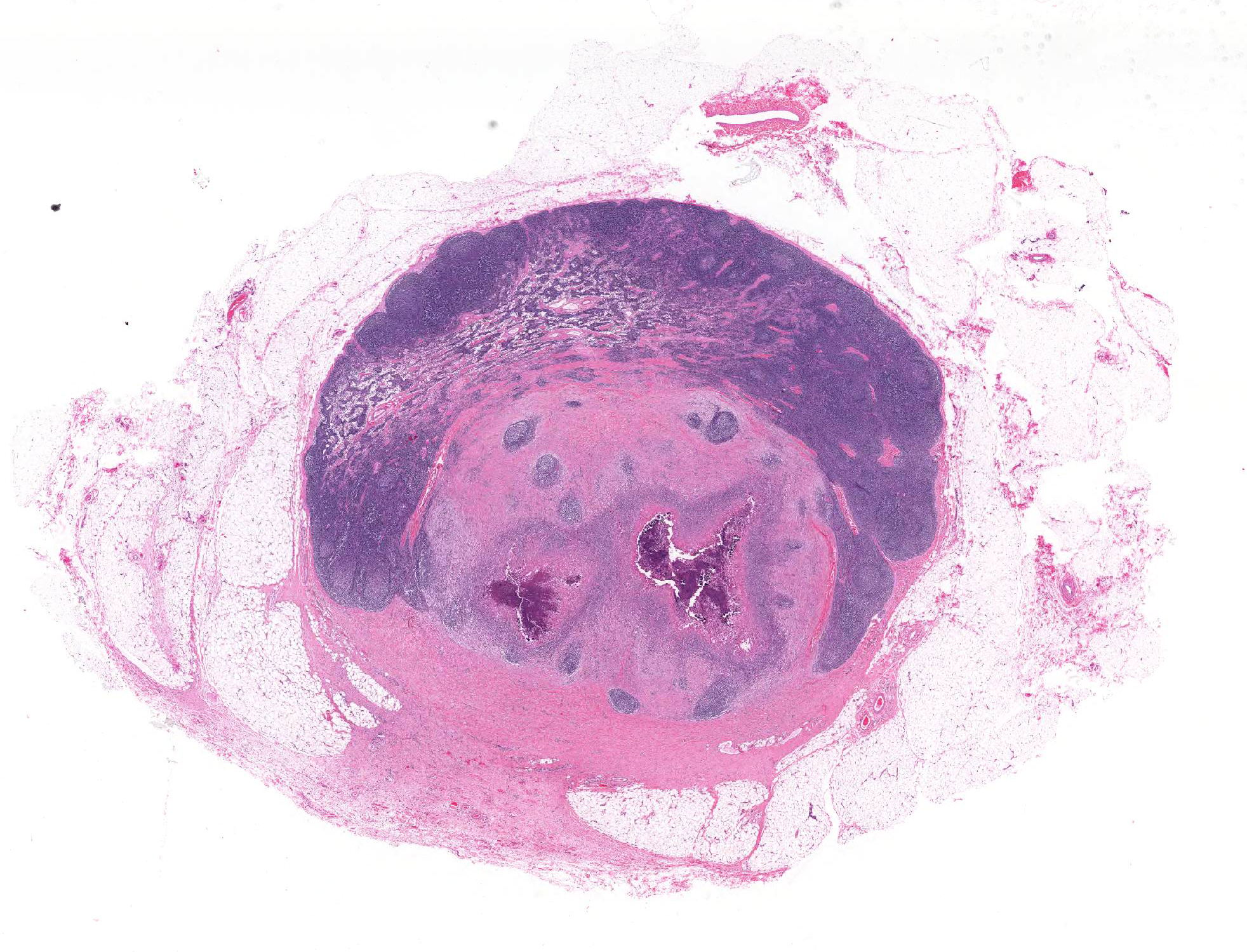
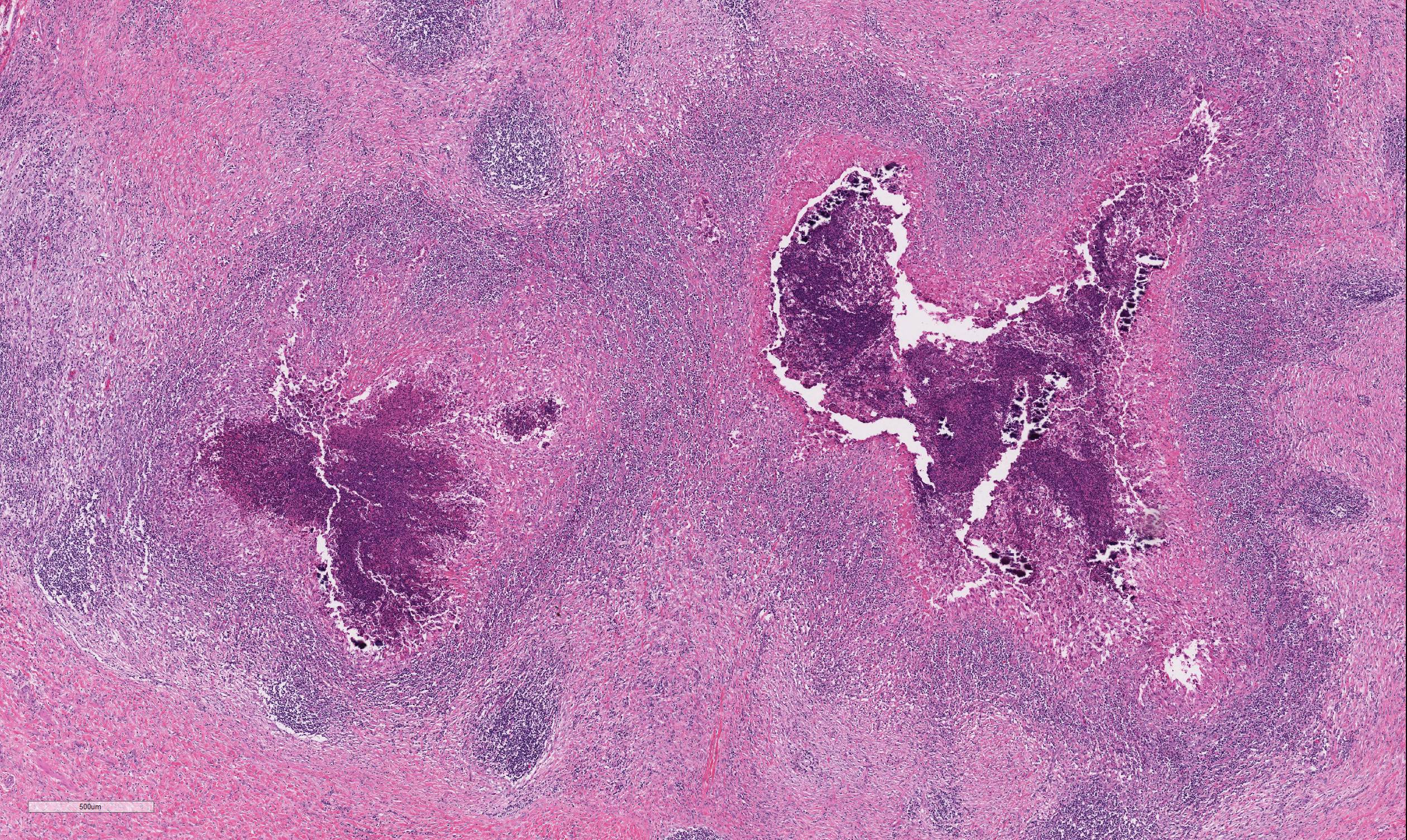
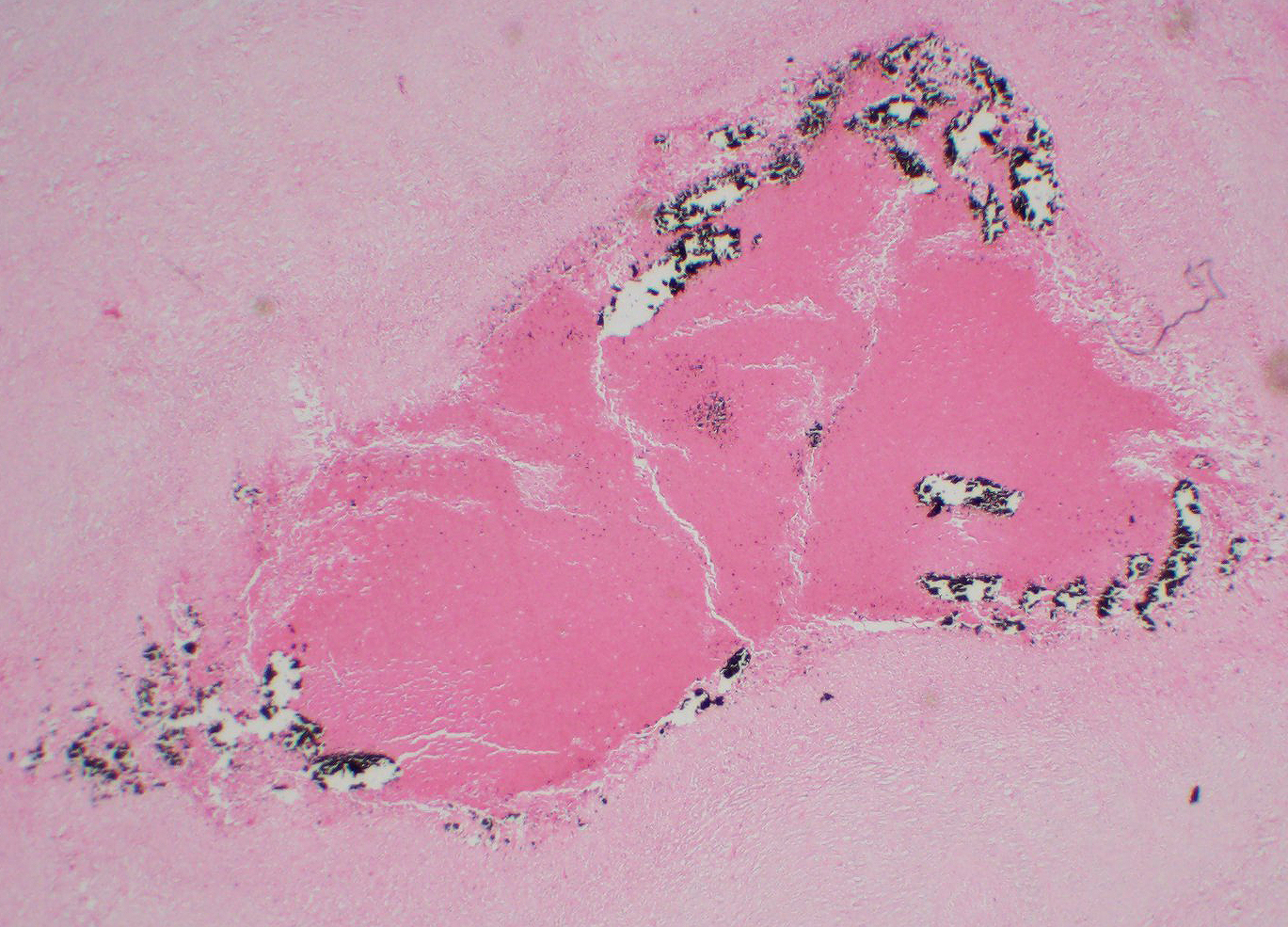
The lymph node is partially effaced by one large or several coalescing discrete, incompletely encapsulated pyogranulomas with a large central core of abundant necrotic cellular debris and degenerate neutrophils with numerous coarse basophilic refractile mineralized concretions. This is surrounded by a layer of moderate numbers of epithelioid and foamy macrophages with occasional multinucleate giant cells, mostly of the Langhans type. Peripheral to this are moderate to large numbers of plasma cells with fewer lymphocytes and macrophages, and rare Mott cells. There is abundant nascent (immature) and mature fibrosis circumscribing the lesion. The lymph node is mildly reactive with pale germinal centers, paracortical lymphoid hyperplasia, and medullary sinus plasmacytosis.
Similar pyogranulomas were present in the liver and lungs (tissue not submitted), often with prominent eosinophil infiltration.
Contributor Morphologic Diagnosis:
Lymph node, left
submandibular, pyogranuloma, focally extensive, chronic, moderate with
mineralization
Contributor Comment: Caseous lymphadenitis is a disease of small ruminants caused by infection with Corynebacterium pseudotuberculosis (C. ovis).1,5 The agent is so named for the gross and histologic similarity of the pyogranulomas to those of tuberculosis, including mineralization and caseation. The organism generally gains entry through cutaneous wounds (often related to shearing or castration), although none were present in this case. Given the lesion location, infection via a wound in the oral cavity is likely. Organisms localize in the local draining lymph node. Infections can also spread internally, commonly to the lungs (as in this case), making this animal unsuitable for research purposes. The organism can survive intracellularly within macrophages due to a leukotoxic surface lipid, and infections tend to be persistent but subclinical. The characteristic green color of the gross exudate is imparted by the accumulation of eosinophils in the lesions. Inspissation of the exudate over time produces the classic lamellated cheesy material.
Corynebacterium pseudotuberculosis is a pleomorphic, gram-positive, non-motile, facultatively anaerobic member of the Actinomycetaceae.1,3 Members of this group are notable for the mycolic acid content of the cell walls and prolonged environmental persistence. Corynebacterium pseudotuberculosis is closely related to C. diptheriae and C. ulcerans. There are two biochemically and genetically distinct biovars of C. pseudotuberculosis, biovar ovis (biotype 1) and biovar equi (biotype 2). The former is typically a pathogen of small ruminants and does not reduce nitrate; the latter is more commonly a pathogen of cattle and horses and does reduce nitrate. Infections in horses include pigeon fever (skeletal muscle abscesses) and ulcerative lymphangitis.4 Virulence factors for this organism include phospholipase D, a sphingomyelin-specific phospholipase, as well as mycolic acids within the cell wall.
Differential diagnosis for this case would include Trueperella (Arcanobacterium) pyogenes, Staphylococcus aureus (botryomycosis), Actinobacillus lignieresii, and Mycobacterium bovis. Gram positive organisms of veterinary importance can be remembered by the acronym SCRAMBLED SCENT, encompassing the genera:
· Staphylococcus
· Clostridium
· Rhodococcus
· Actinomyces
· Mycobacterium
· Bacillus
· Listeria
· Erysipelothrix
· Dermatophilus
· Streptococcus
· Corynebacterium
· Enterococcus
· Nocardia
· Trueperella
Subcutaneous reactions to clostridial vaccines, particularly around the neck or scapula, can also be mistaken for CLA.
Contributing Institution:
Department of Comparative Medicine
Penn State Hershey Medical Center
http://www.hmc.psu.edu/comparativemedicine/
JPC Diagnosis: Lymph node: Pyogranuloma, focal.
JPC Comment:
The contributor
gives a concise review of Corynebacterium pseudotuberculosis infection
in small ruminants. This gram-positive facultative intracellular pathogen,
which may exhibit pleomorphism in tissue, such as coccoids and filamentous rods,2
is best known for abscess formation in small ruminants, also affects a wide
range of other species, including horses (as previously mentioned), cattle,
camelids, deer, and humans. The bacterium was first identified by the French
bacteriologist Edward Nocard from a cow, and three years later, by the
Bulgarian Hugo von Priesz from a ewe. For the next thirteen, it was referred
to as the Priesz-Nocard
bacterium, where upon it was renamed Bacillus
pseudotuberculosis in the atlas by prominent German bacteriologists Lehman
and Neuman. In the 1923 first edition of Bergey´s Manual of Determinative
Bacteriology it was placed in the genus of Corynebacterium, where it
remains today. It was at that time called Corynebacterium ovis, but
after discovered to cause infection in a number of species, reverted back to C.
pseudotuberculosis in 1948, by the sixth edition of that manual.1
In sheep,
infection usually follows wound infection, and at shearing time, infected
abscesses may be punctured, bacterial liberated in a common dip tank, and the
bacterium may invade shearing wounds on other sheep or even penetrate intact
skin. In addition to direct contact, the disease may also be spread by sheep
with established respiratory infections coughing on the open wounds of penmates.2
If the bacteria are not confined to and eliminated from the skin, the infection
may progress to draining nodes.6 Mature abscesses in the lymph nodes
of sheep may achieve a greenish lamellated In sheep, especially older sheep, the infection may progress from peripheral
lymph nodes to internal nodes or organs, especially the lungs, resulting in
chronic systemic disease referred to as the "thin ewe syndrome" (apparently
more common in the US than in other countries)1. The presence of
abscesses within nodes and carcass meat generally results in condemnation,
which, in countries which utilize lamb for religious celebration, may result in
a loss of $200 per animal to the purveyor. Many countries have strict
importation guidelines regarding contamination of small ruminants with this
bacterium. The importance of CLA vaccination is exemplified by the decrease in
CLA in Australia alone - in 1973, CLA among sheep in Western Australia was
estimated at 58%; following introduction of a CLA vaccine in 1983, similar
studies recorded a prevalence of 45%, which in turn dropped to 20% in 2002.1 In goats, lesions are more often severe, and abscesses tend to cluster in the
nodes of the face and neck. The liquid nature of abscessed nodes is
reminiscent of melioidosis (Burkholderia pseudomallei infection) in this
species.6 Other lesions associated with C. pseudotuberculosis in
sheep and goats include mastitis (presumably resulting from local spread from
abscessed supramammary nodes)1 and polyarthritis in young lambs, but
overall, the disease is rarely fatal, even in prolonged infection.6
Another disease caused by C. pseudotuberculosis in the horse is equine
folliculitis and furunculosis (also referred to as equine contagious acne, ,
equine contagious pustular dermatitis and "Canadian horsepox"). It is most often
seen at points of contact with tack in animals with pre-existent seborrheic
dermatitis, and likely represents secondary invasion by bacteria spread on
contaminated tack.1 The moderator, who did her PhD studying this agent, reviewed the various pathogenic factors which allow it to cause disease in a range of ruminants and horses. Like other higher bacteria, the presence of mycolic acid in the wall of the bacterium, which allows it to resist digestion in phagocytes also lends a unique property to colonies in culture â the ability to slide easily across the plate, known as "shuffleboard" coloniesâ. Another virulence factor, phospholipase D, is important for tissue invasion. In sheep and goats, phospholipase D allows the bacteria to invade through sphingomyelin-containing endothelium, allowing for extensive intravascular spread of the bacterium. In the biovar infecting horses, the bacterium do not possess sufficient phospholipase D for intravascular spread, and must be introduced in a wound, resulting in a slow progressive lymphangitis.
The moderator also described the synergistic hemolysis-inhibition titers (with a rather rude acronym) which were used in the 1980s for diagnosis of occult infection of C. pseudotuberculosis in small ruminants in Brazil. The synergistic hemolysis-inhibition test detects antibodies to an exotoxin of C. pseudotuberculosis by the inhibition of a synergistic hemolysis between the toxins of C. pseudotuberculosis and R. equi.
References: 1. Baird GJ,
Fontaine MC: Corynebacterium pseudotuberculosis and its role in ovine caseous
lymphadenitis. J Comp Pathol 2007:137(4):179-210. 2. Dorella FA, Pacheco LGC, Oliveira
SC, Miyoshi A, Azevedo. Corynebacterium pseudotuberculosis:
microbiology, biochemical proterties, pathogenesis and molecular studies of
virulence. Vet Res 2016; 17:201-218. 3 Soares SC, Silva A, Trost E, Blom
J, Ramos R, Carneiro A, et al.: The pan-genome of the animal pathogen
Corynebacterium pseudotuberculosis reveals differences in genome plasticity
between the biovar ovis and equi strains. PLoS One 2013:8(1):e53818. 4 Valentine BA, McGavin MD: Skeletal
Muscle. In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary
Disease, Fourth Ed. St. Louis, MO: Elsevier; 2007: 973-1039. 5 Valli VEO: Hematopoietic System.
In: Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic Animals.
Fifth ed. Edinburgh: Saunders Elsevier; 2007: 107-324. 6. Valli VEO, Kiupel M. Bienzle D.
In. Maxie MG, ed. Jubb, Kennedy, and Palmer's Pathology of Domestic
Animals. Sixth ed. Edinburg: Saunders, Elesevier, 2016, pp 204-208. onion-skin appearance
due to
recurrent and alternating episodes of suppuration and encapsulation; in goats,
the abscesses tend to demonstrate a more liquefied appearance. When the
infection reaches the lymph nodes, the condition is considered persistent and
lifelong.1