Wednesday Slide Conference, Conference 11, Case 3
Signalment:
6-month-old, female intact, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mouse (Mus musculus). This mouse strain has the Prkdc and interleukin 2 receptor (Il2rg) mutations, which results in phenotype that lacks numerous immune components including T cells, B cells, natural killer cells, and have deficient signaling for 6 cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21). 8
History:
The mouse was part of experiment in which a NSG mouse shedding Chlamydia muridarum was cohoused with four naïve NSG mice. The mouse was lethargic, lost weight, and had a hunched posture and an increased respiratory effort.
Gross Pathology:
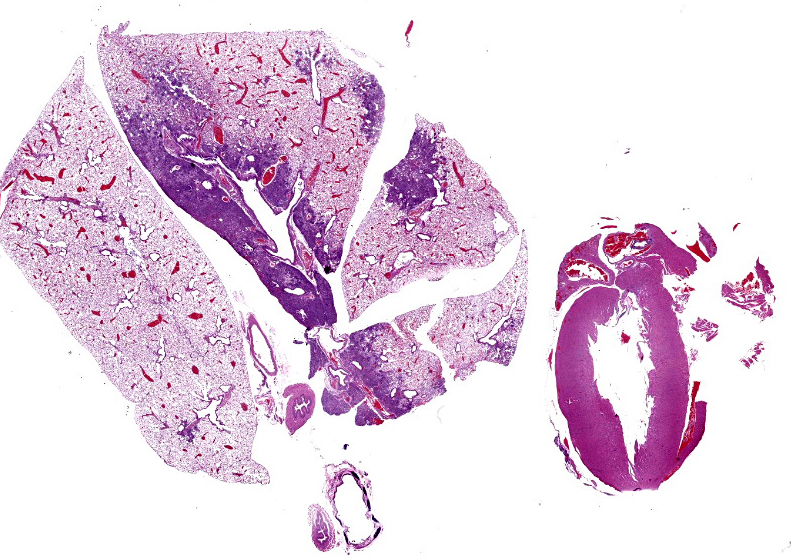
The right cranial, middle, and accessory pulmonary lobes were diffusely pale pink to white and atelectatic.
Laboratory Results:
CBC with manual differential showed leukocytosis characterized by mild to moderate neutrophilia and mild monocytosis.
Aerobic and anaerobic cultures of the lung were both negative.
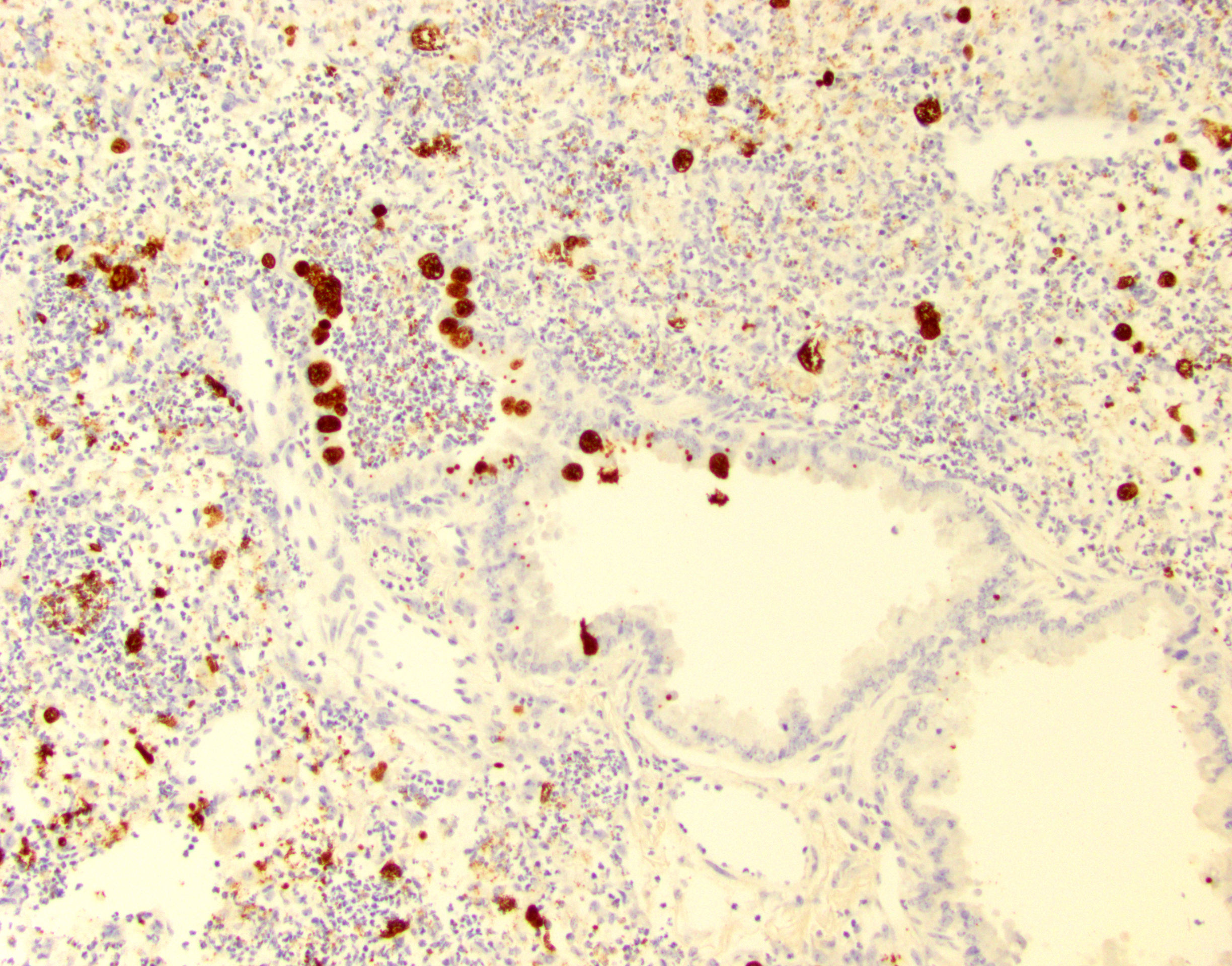
IHC for Chlamydia MOPM-1 showed strong and specific detection of Chlamydia antigen in bronchiolar and alveolar epithelial cells and areas of inflammation.
qPCR for Cm 23S rRNA: Positive in lung
Microscopic Description:
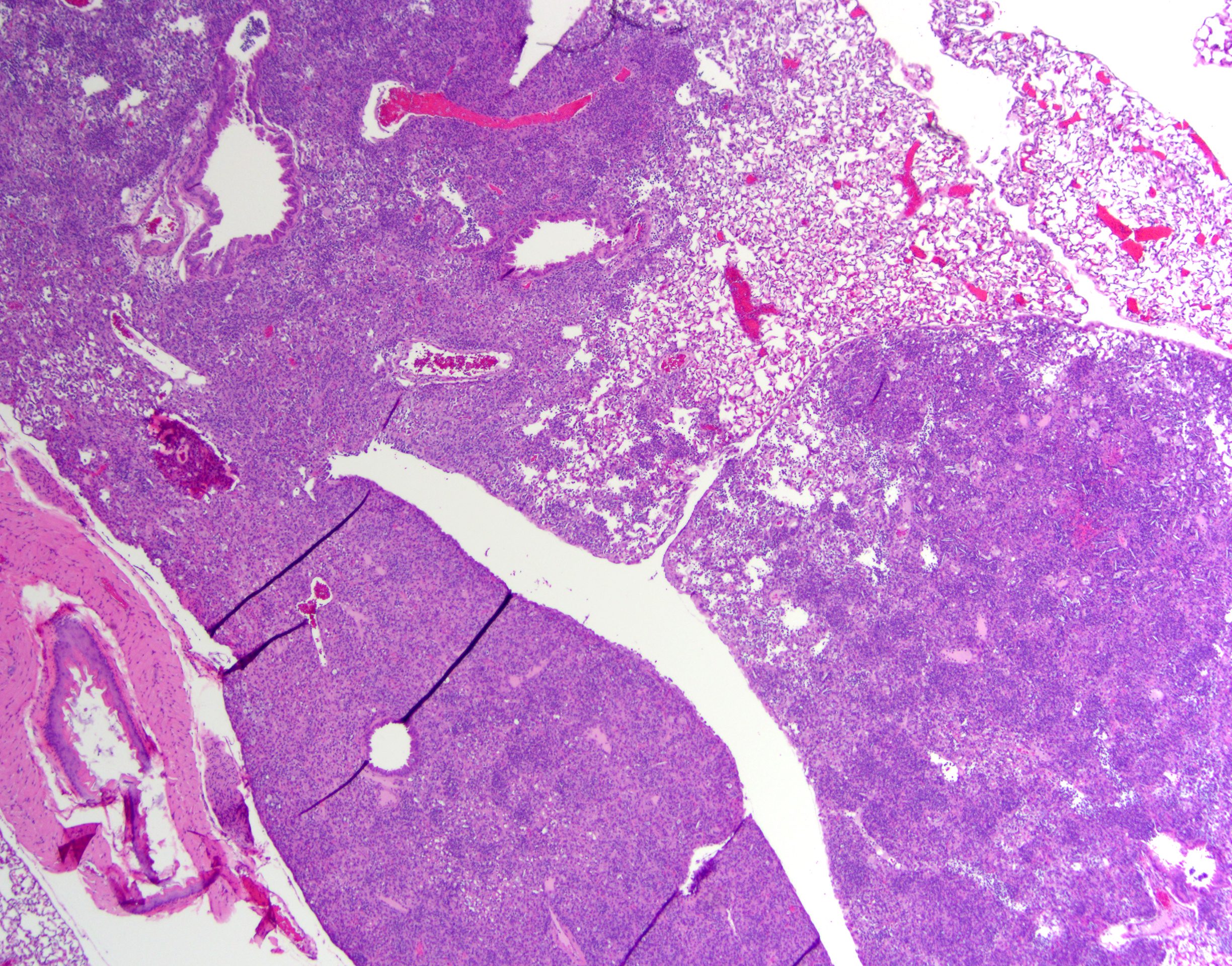
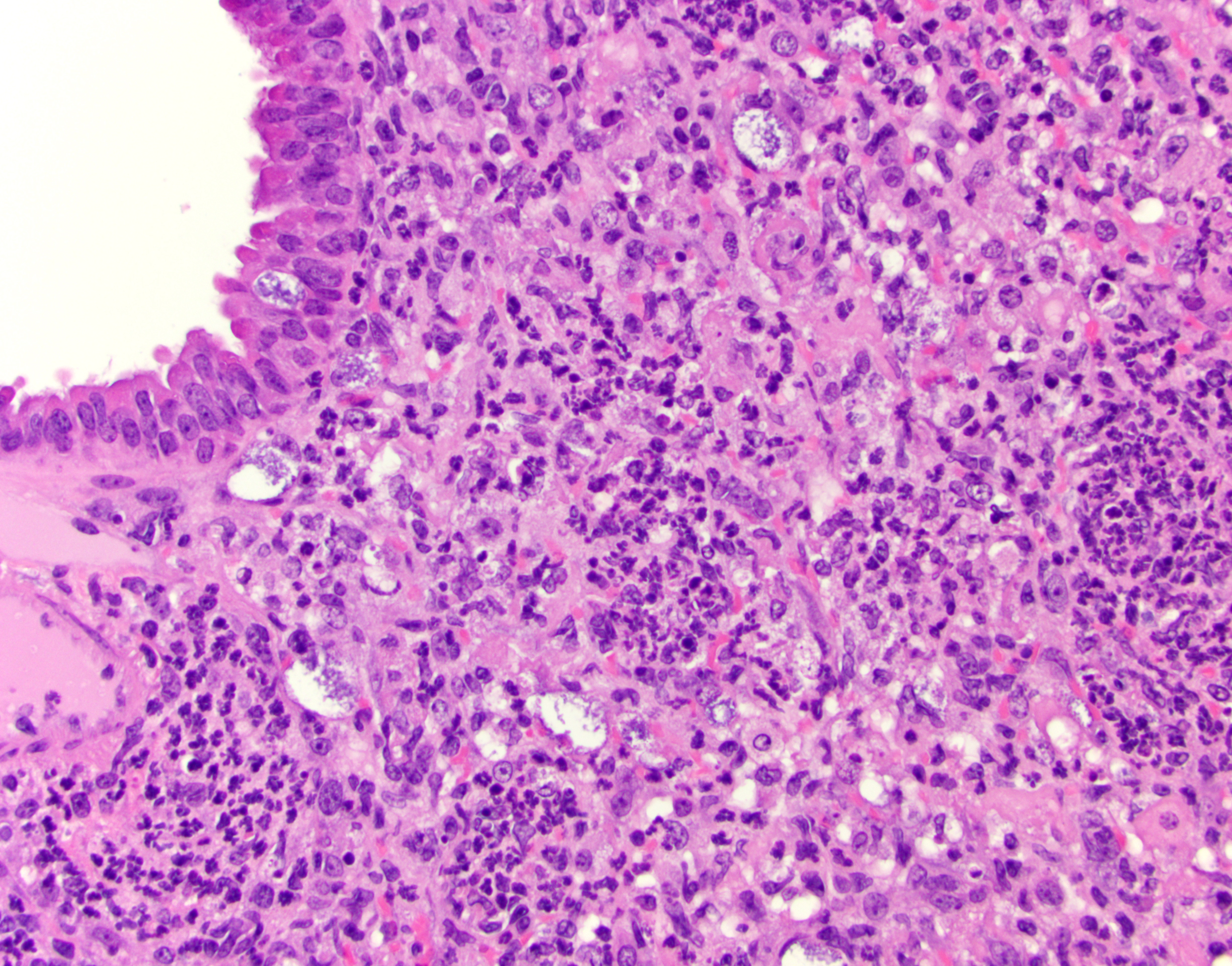
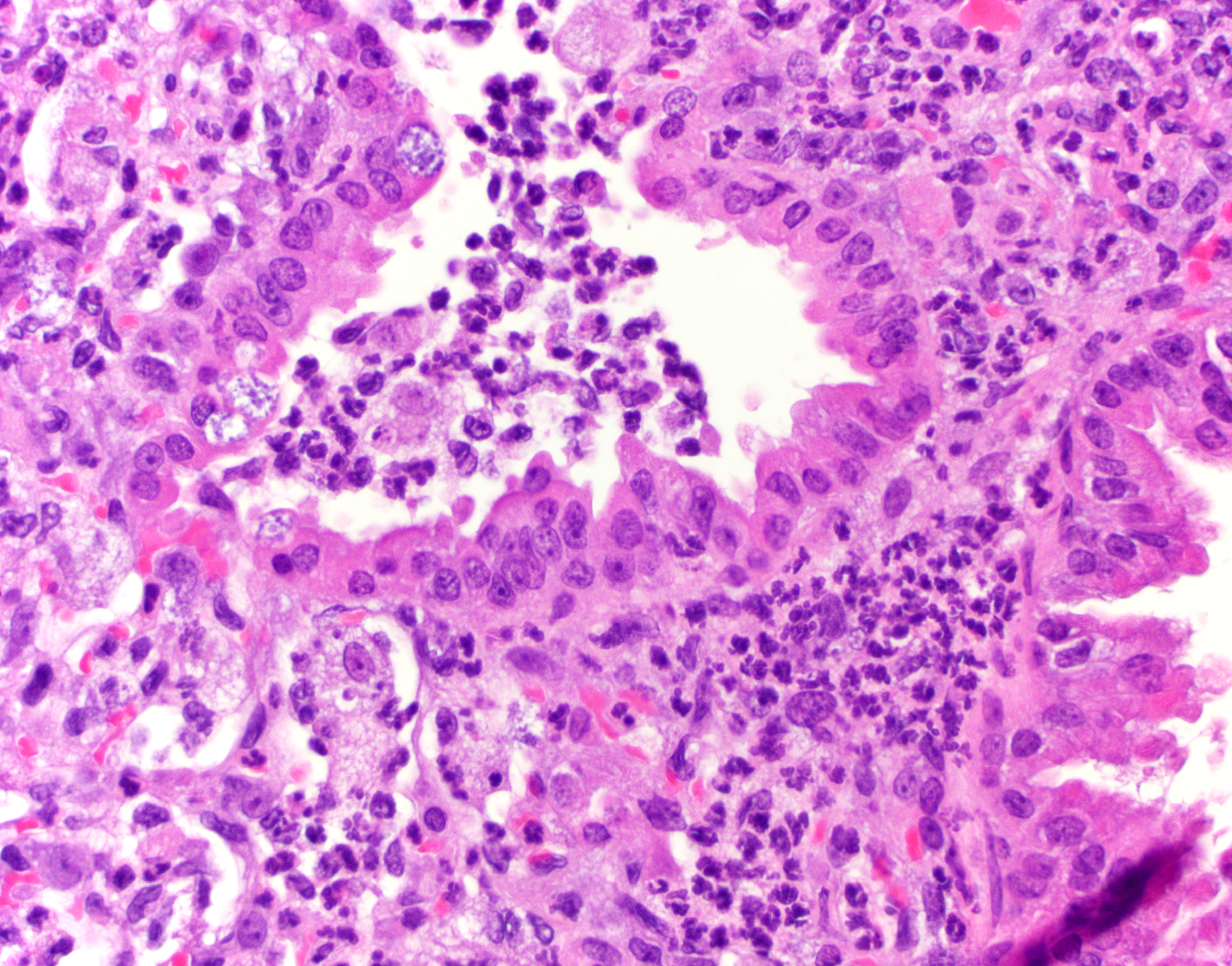
Lung (right and left pulmonary lobes): Affecting approximately 40-45% of the airways in the right pulmonary lobes, there are multifocal to coalescing dense areas of leukocytic cell infiltration mixed with proteinaceous exudate and necrotic cellular debris. Multifocally, alveolar spaces are collapsed and filled with numerous neutrophils and degenerate neutrophils admixed with foamy macrophages, edema, fibrin, karyorrhectic debris and fewer linear clear acicular clefts (cholesterol clefts), and hemorrhage. Alveolar septa are lined by elongated epithelial cells and attenuated necrotic alveolar wall admixed with alveolar macrophages and vacuolated cells containing numerous round basophilic organisms measuring approximately 0.5 to 1.0μm in diameter (Chlamydia elementary and reticular bodies). The adjacent bronchioles are filled by moderate amounts of proteinaceous fluid admixed with neutrophils, karyorrhectic debris, pyknotic cells, and fibrin. The bronchiolar epithelium is segmentally effaced by areas of necrosis and proteinaceous exudate and often contains degenerate bronchiolar epithelial cells with intracytoplasmic vacuoles filled with Chlamydia elementary and reticular bodies (Chlamydia inclusions). Blood vessels are congested and lined by plump endothelial cells with tethered neutrophils. The left pulmonary lobe is multifocally affected by small clusters of neutrophils and foamy macrophages admixed with edema and hemorrhage, predominantly affecting alveoli. Chlamydia inclusions are occasionally noted in the left pulmonary lobe and often observed in the adjacent extrapulmonary bronchi.
Contributor’s Morphologic Diagnosis:
Lung: Bronchointerstitial pneumonia histiocytic and neutrophilic, chronic, multifocal to coalescing, moderate to severe, with edema, fibrin, necrotic debris, alveolar histiocytosis, numerous intracytoplasmic Chlamydia inclusions.
Bronchi, tracheal bifurcation: Multifocal intraepithelial Chlamydia inclusions.
Contributor’s Comment:
Chlamydia muridarum (Cm) was recently reported as prevalent in research mouse colonies, affecting between 14% and 33% of noncommercial institutions.5 Cm infections in mice were initially described in the 1930s and 1940s when this bacterium was discovered by accident while inoculating mice with various viruses by transferring lung homogenates.4,7 In 2021, our group detected Cm inclusions associated with peribronchiolar lymphocytic and plasmocytic aggregates in immunocompetent GEM mouse strains.4 The case presented in this submission was part of an investigation to evaluate the impact of Cm infection in severely immunocompromised mice (NSG) after cohousing with Cm shedding, naturally infected immunocompetent mice and/or their soiled bedding for 4 weeks.9 All 19 NSG mice developed pulmonary lesions consistent with bronchointerstitial pneumonia and/or bronchiolitis with numerous intraepithelial Chlamydia inclusions bronchioles and alveoli.9 Since 2021, our laboratory has reported that Cm infections can be associated with spontaneous clinical disease and pulmonary and/or urogenital pathology in NSG mice and in two genetically engineered mouse (GEM) strains, Il12rb2 knockout and STAT1 knockout mice, with impaired interferon-γ signaling and Th1 CD4+ T cell responses.4,9 As a result of these investigations, testing for Cm has been added to routine health surveillance testing panels in immunocompromised mouse colonies in our institution and commercial breeding colonies in other institutions in the U.S.
Chlamydiae are gram-negative obligate intracellular bacteria with an extensive host range at the genus level, but high host specificity at the species level.3,7 Cm is the only natural chlamydial pathogen of mice and has been classically used to model the sexually transmitted Chlamydia trachomatis infection of humans.3 Cm exhibits a biphasic life cycle involving a nonreplicating and infectious ‘elementary body’ and a replicating and noninfectious ‘reticulate body.3,7 Elementary bodies enter the host mucosal epithelial cells and incorporate into a membrane-bound compartment, termed an inclusion body. These elementary bodies then differentiate into reticular bodies. The reticular bodies replicate within the inclusions before reverting into elementary bodies, as which they can then be released and infect nearby cells.3,7 Natural transmission is via the fecal-oral route, and the gastrointestinal tract is often the natural site of colonization.14,15 It is thought that pulmonary lesions and colonization can also be acquired through aspiration of inhalation of the organism.5,9
The diagnosis of Cm infection in laboratory mice can be confirmed by Cm MOMP-1 IHC and/or qPCR or ISH using Cm-specific primers and probes, respectively.9 The bronchointerstitial pneumonia and numerous intralesional CI inclusions are characteristic microscopic changes seen in spontaneous and/or experimental Cm infections in NSG mice.5,9 Cm inclusions are rarely noted in infected lungs from immunocompetent mice5 and requires the use of ancillary tests for confirmation. Differential diagnoses in immunodeficient mice include bronchopneumonia associated with Mycoplasma pulmonis or Filobacterium rodentium (“CAR bacillus”) and primary viral infections with Sendai virus or Pneumonia Virus of Mice (PVM), granulomatous interstitial pneumonia associated with Pneumocystis murina, and bronchopneumonia associated with other opportunistic bacterial agents.1,10
The bronchointerstitial pneumonia and bronchiolitis seen in Cm infected NSG mice shared similarities with pulmonary lesions seen in the acute phase of Cm infection in previous studies using BALB/c and Tlr2 knockout mice following intranasal challenge.2,12 Pulmonary lesions in Il12rb2 knockout and STAT1 knockout mice naturally infected with Cm are characterized by lymphoplasmacytic and histiocytic inflammation in bronchioles and peribronchiolar and perivascular spaces.4 Cm inclusions are seen in bronchiolar epithelial cells, but these inclusions are less abundant than the inclusions seen from NSG-infected lungs.4,9 The severity of the pulmonary lesions seen in NSG mice is likely exacerbated by the absence of CD4+ T and NK cells in this mouse strain.9 These immune cells are important producers of interferon-gamma (IFN-γ), which is an essential effector cytokine involved in the resolution of Cm infections in experimental models of murine chlamydiosis.13
Cm has shown a tropism for mucosal epithelial cells, which serves as a niche for intracellular survival, spread from cell to cell, and immune modulation.3,6 Cm inclusions in NSG mice were observed in epithelial cells from the nasopharynx, nasal cavity, trachea, eustachian tube, bronchi/bronchioles, oviducts, uterus, and small and large intestines.9 Among these tissues Cm extensively colonized the small and large intestinal epithelium, without eliciting histopathological changes in the mucosa.9 Given that NSG mice do not have functional adaptive immune system,8 Cm likely establishes long-lasting colonization in the intestines and persistent shedding of elementary bodies in feces.
Contributing Institution:
Laboratory of Comparative Pathology; Memorial Sloan Kettering Cancer Center, Weill Cornell Medicine, Hospital for Special Surgery, and The Rockefeller University
417 E. 68th St., ZRC-940
New York, NY 10065
https://www.mskcc.org/research/ski/core-facilities/comparative-medicine-pathology-0
JPC Diagnosis:
Lung: Pneumonia, bronchointerstitial, necrotizing, neutrophilic and histiocytic, subacute, multifocal to coalescing, marked, with numerous intraepithelial bacterial inclusions.
JPC Comment:
This case was recently published in Veterinary Pathology11 and the submitted slides are just as good in person as they are “on paper”. The contributor provides a great summary of the paper in their comments, but we invite readers to read it in its entirety in conjunction with this case.
The distribution of Chlamydia in this case is generous and the lack of IFN-γ in these mice allowed conference participants to appreciate inclusion bodies readily on H&E, though they stained strongly with PAS as well. Dr. Alves also performed ISH for Chlamydial RNA using a C. trachomatis probe which cross-reacted and labeled Cm-infected cells strongly. Additionally, alveolar macrophages (labeled with a F4/80 IHC marker) were assessed, though we did not see actual Chlamydial inclusion bodies within macrophages. Macrophage replication is a feature of C. trachomatis infection but was not observed here.
Conference participants also touched on several ancillary changes. In less affected regions of lung, there were numerous alveolar macrophages which was interpreted as alveolar histiocytosis. This is a common background finding in knockout mice. Additionally, the large neutrophilic response in an animal lacking other tools to respond to a pathogen is not surprising – participants noted good examples of leukocyte margination in blood vessels. Though there was also a focus on inflammatory cells along the pericardium, these were interpreted as mast cells as they notably lacked PAS-staining or ISH probe labeling.
There were some indications of chronicity in section. Notably, there is a large, mature thrombus in a pulmonary vein. Additionally, the bronchiolar epithelium is markedly hyperplastic whereas in unaffected sections the epithelium is quite thin. The accumulation of cholesterol crystals is also suggestive of significant loss and accumulation of cell membrane components. In line with the contributor’s purported infection timeline11 we believe that the time course of these changes is at least subacute.
References:
- Barthold SWP, D.H.; Griffey, S.M. Chapter 1: Mouse In: Barthold SWP, D.H.; Griffey, S.M., ed. Pathology of Laboratory Rodents. Fourth ed.: Wiley-Blackwell; 2016:14-81.
- Beckett EL, Phipps S, Starkey MR, Horvat JC, Beagley KW, Foster PS, Hansbro PM. TLR2, but not TLR4, is required for effective host defence against Chlamydia respiratory tract infection in early life. PLoS One. 2012;7: e39460.
- Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol. 2016;14: 385-400.
- Mishkin N, Miranda IC, Carrasco SE, et al. Chlamydia muridarum Associated Pulmonary and Urogenital Disease and Pathology in a Colony of Enzootically Infected Il12rb2 Deficient and Stat1 Knockout Mice. Comp Med. 2024;74: 121-129.
- Mishkin N, Ricart Arbona RJ, Carrasco SE, et al. Reemergence of the Murine Bacterial Pathogen Chlamydia muridarum in Research Mouse Colonies. Comp Med. 2022;72: 230-242.
- Perry LL, Hughes S. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun. 1999;67: 3686-3689.
- Rank RG. Chlamydial diseases. In: Fox JGD, M.T.; Quimby, F.W.; Barthold, S.W.; Newcomer, C.E.; Smith, A.I. , ed. The mouse in biomedical research. Burlington (MA): Elsevier; 2007:326-344.
- Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174: 6477-6489.
- St Jean SC, Ricart Arbona RJ, Mishkin N, et al. Chlamydia muridarum infection causes bronchointerstitial pneumonia in NOD.Cg-Prkdc(scid)Il2rg(tm1Wjl)/SzJ (NSG) mice. Vet Pathol. 2024;61: 145-156.
- Stair MI, Carrasco SE, Annamalai D, et al. The Epidemiology of Invasive, Multipleantibiotic-resistant Klebsiella pneumoniae Infection in a Breeding Colony of Immunocompromised NSG Mice. Comp Med. 2022;72: 220-229.
- St Jean SC, Ricart Arbona RJ, Mishkin N, et al. Chlamydia muridarum infection causes bronchointerstitial pneumonia in NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. Veterinary Pathology. 2024;61(1):145-156.
- Virok DP, Raffai T, Kokai D, et al. Indoleamine 2,3-Dioxygenase Activity in Chlamydia muridarum and Chlamydia pneumoniae Infected Mouse Lung Tissues. Front Cell Infect Microbiol. 2019;9: 192.
- Winner H, Friesenhahn A, Wang Y, Stanbury N, Wang J, He C, Zhong G. Regulation of chlamydial colonization by IFNgamma delivered via distinct cells. Trends Microbiol. 2023;31: 270-279.
- Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis. 2013;68: 88-95.
- Zhong G. Chlamydia overcomes multiple gastrointestinal barriers to achieve long-lasting colonization. Trends Microbiol. 2021;29: 1004-1012.