Signalment:
7-year-old, female spayed, domestic shorthair cat (
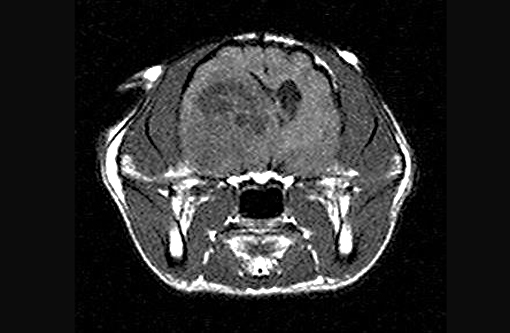
Felis cats).The cat had a history of acute onset of seizures with concurrent circling to right and a left menace deficit. Based on the MRI characteristics of rim enhancement and large amounts of perilesional edema, right thalamic abscess, neoplasia and granuloma were the top differential diagnoses. There was initially a good response to antibiotics and phenobarbital, and the cat was lost to follow-up for several months. The cat represented 14 months later, and demonstrated no response to repeat steroids, therefore, the owner elected euthanasia. An MRI was obtained prior to euthanasia revealing a large, right thalamic mass.
Gross Description:
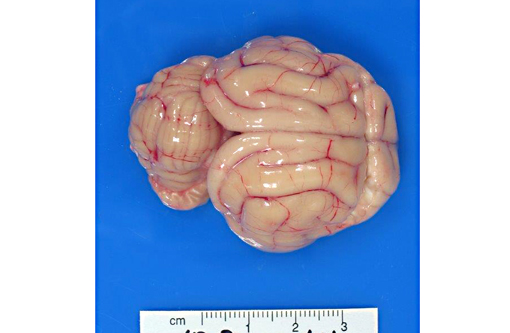
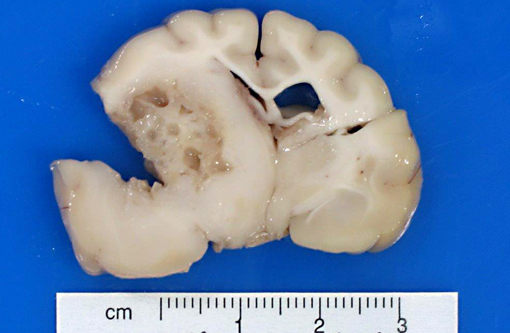
External examination of the brain from the dorsal and ventral aspects reveals asymmetric enlargement of the right cerebrum, involving the right pyriform lobe. Upon sectioning of the fixed brain, there is a 2 cm diameter, large, firm, tan to light yellow, gelatinous, cystic mass extending from the right cerebrum to the midbrain. The mass compresses the right lateral and third ventricles, causing a midline shift, with marked expansion of the pyriform lobe and obliteration of basal nuclei.
Histopathologic Description:
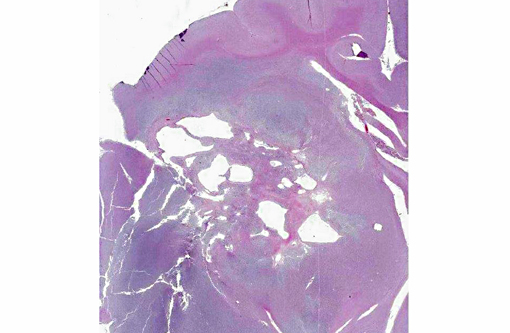
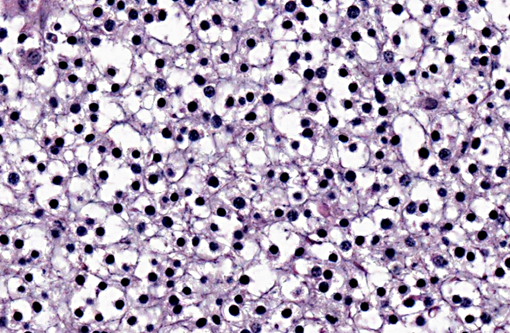
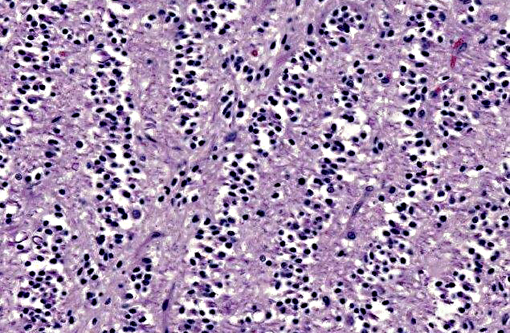
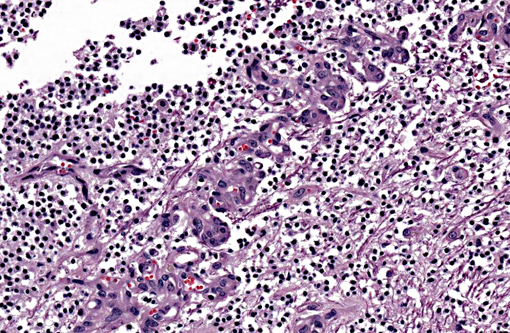
A section of the brain including the cerebral cortex and thalamus is examined at the level of the body and crura of fornix, basal nuclei, third ventricle and pituitary gland (not all slides contain pituitary gland). Within the thalamus, white matter of the pyriform lobe and cerebral cortex of the temporal lobe, extending to involve the grey matter, there is a poorly demarcated, infiltrative, densely cellular neoplasm, consisting of round to polygonal cells forming sheets and cords amongst an eosinophilic fibrovascular to amphophilic myxomatous stroma. Neoplastic cells are mildly pleomorphic, with variably distinct cell borders, clear (perinuclear halo) to lightly stained vacuolated eosinophilic cytoplasm, and round, central nuclei. Nuclei are typically hyperchromatic, with indistinct chromatin and nucleoli. Mild anisokaryosis is observed, up to two fold. There are zero mitoses in ten high power fields. In some regions of the neoplasm, cell borders are prominent (fried egg or honeycomb pattern). Multifocally, vessels are conspicuous, characterized by prominent endothelial hypertrophy and proliferation (glomeruloid vessels). Clear, cystic spaces are dispersed throughout the center of the neoplasm, and a focus of hemorrhage is present in some slides. Within these regions, the white matter parenchyma is rarefied (edema), containing swollen axons (spheroids) and glial cells, including Gitter cells and gemistocytic astrocytes. Remnant neurons from the infiltrated neuroparenchyma and reactive glial cells are scattered throughout the neoplastic populations. In the section of pituitary gland, there is mild acidophil hyperplasia. Mild endothelial hypertrophy is present in the small vessels of the adjacent and contralateral cerebral cortex.
Morphologic Diagnosis:
Brain (thalamus): Oligodendroglioma with intralesional and peripheral edema and gliosis.
Pituitary gland: Mild acidophil hyperplasia.
Lab Results:
Cytology of the mass did not reveal any inflammatory cells
Condition:
Oligodendroglioma
Contributor Comment:
Oligodendroglioma is a primary central nervous system neoplasm composed of oligodendrocytes, the myelin producing cells of the central nervous system (CNS). In cats, glial tumors are reported to be the fourth most common CNS neoplasm, preceded by meningioma, lymphoma and, in one study, pituitary neoplasms.(5,6) In a retrospective study of 160 cases of feline intracranial neoplasia, six oligodendrogliomas were reported, the median age for which was 9.3 years. Seizures were the most common clinical sign, along with altered consciousness, aggression, circling and ataxia in 2 cats.(6) A study analyzing tumor types and seizure patterns in 61 cats found that generalized seizures (vs partial seizures) were most common in cats with intracranial neoplasia. In that study, the highest incidence of seizures occurred with astrocytomas (33%), with a high overall incidence of seizures with all glial cell tumors (26.7%).(5) Oligodendrogliomas can occur in all areas of the white matter of the cerebrum, brainstem, and interventricular septum.(3,4,7) Feline oligodendrogliomas in the aforementioned larger case series were most commonly found in the temporal lobes, and all were located in the ventral portion of the brain, consistent with previous reports of this tumor in the deeper structures of the cerebral hemisphere.(5) In dogs, this tumor is more common in brachycephalic breeds.(3,4,6,7)
Macroscopic features of oligodendrogliomas range from soft and gelatinous to firm and they are frequently grey to pink-red, varying from well demarcated to poorly demarcated.(3,4,7) Neoplastic oligodendrocytes are typically uniform in cellular and nuclear size and shape, with rare mitoses. Characteristic features of this neoplasm include the clear to lightly stained cytoplasm (perinuclear halo) and a distinct cell membrane.(3,4,7) Autolysis is thought to lend to the fried egg or honeycomb appearance of the neoplastic cells.(1,3) Mucinous cystic degeneration, edema, cavitation and rarely mineralization can occur, and necrosis is rare. Prominent branching capillary proliferation forms a chicken wire vascular pattern.(3,4) At the margins of the tumor, neoplastic cells may be arranged in chains (as in this case), reminiscent of interfascicular oligodendrocytes.(3,4,7) Perivascular cuffing and satellitosis of neurons with neoplastic cells may occur in infiltrated grey matter.(4) Anaplastic oligodendrogliomas are characterized by nuclear pleomorphism, high cellularity, a high mitotic index, prominent vascularization, intratumoral necrosis and rarely, calcification.(1,4,7) The initial MRI findings of rim enhancement and perilesional edema could not differentiate neoplasia from inflammatory conditions including abscess and granuloma. A report detailing clinical and pathologic features of oligodendrogliomas in two cats found heterogenous contrast enhancement and hyperintensity of proton weighted (PW) and T2 weighted images and either iso or hypointensity on T1 weighted images in both cases.(1) Post contrast ring enhancement has been reported with CT scans of canine oligodendrogliomas.(1)
Some human oligodendrogliomas express S-100 protein and the Leu-7 marker, but this expression pattern is not specific for oligodendrogliomas.(3) Immunoreactivity for GFAP may indicate the presence of early transitional glial cell forms.(3) Normal oligodendrocytes do not express GFAP, but immunoreactivity for GFAP can be demonstrated in up to 50% of human oligodendrogliomas, which may be due to reactive gemistocytic astrocytes.(1) A case series of two cats with oligodendrogliomas identified minigemistocytic astrocytes or gliofibrillary oligodendrocytes in one cat. These cells may represent a transitional form between neoplastic oligodendrocytes and astrocytes and appear identical to oligodendroglioma cells, but have a thin perinuclear rim of GFAP positive cytoplasmic staining.(1) GFAP immunohistochemistry was not performed in this case. Detection of smooth muscle actin expression in the glomeruloid vascular proliferation is a distinctive feature of anaplastic oligodendrogliomas of dogs.(1)
JPC Diagnosis:
Brain, cerebrum at level of midbrain: Oligodendroglioma
Conference Comment:
The contributors adept summary includes a brief discussion of patterns of immunohistochemical expression in oligodendrogliomas. Recently, Ide et al. explored several markers to identify characteristic immunohistochemical profiles for each of the eight types of canine neuroepithelial tumors (i.e. astrocytic tumors, oligodendroglial tumors, other gliomas, ependymal tumors, choroid plexus tumors, neuronal and mixed neuronalglial tumors, embryonal tumors, and pineal parenchymal tumors).(2) They found that in oligodendrogliomas, the majority of cells were positive for doublecortin (DCX) but negative for glial fibrillary acidic protein (GFAP). Furthermore, benign oligodendrogliomas contained few nestin-positive cells compared to anaplastic oligodendrogliomas, which contained many nestin-positive cells. Two anaplastic oligodendrogliomas displayed positivity for Beta III tubulin. All cases of oligodendrogliomas were negative for neurofilament (NF), cytokeratin AE1/AE3, or cytokeratin 18. DCX plays a major role in neuroblast migration during cerebral cortex development. GFAP is an intermediate filament protein of mature astrocytes; nestin is an intermediate filament protein of immature astrocytes; NF is a marker for mature neurons; beta III tubulin is used to identify early neuronal differentiation; cytokeratin AE1/AE3 is used to identify many high- and low-molecular weight cytokeratins; and cytokeratin 18 is a simple epithelial cytokeratin.(2) As for expression of proliferation/ apoptosis markers in oligodendrogliomas, the following results were found: Generally, the percentage of Ki-67-positive cells were low, with the exception of two cases; p53 expression by more than 10% of tumor cells was observed in one case; there were higher numbers of Bcl-2 positive tumor cells in comparison to Bcl-xL-positive cells, and no cells showed positivity for epidermal growth factor receptor (EGFR), c-erbB2 (aka HER-2 or neu), phosphoextracellular signal-regulated kinase 1/2 pERK1/2, phospho-Akt (pAkt), or cleaved caspase 3.(2)
References:
1. Dickinson PJ, Keel MK, Higgins RJ, Koblik PD, Lecouteur RA, Naydan DK, et al. Clinical and pathologic features of oligodendrogliomas in two cats.
Vet Pathol. 2000;37:160-167.
2. Ide, Uchida K, Kikuta F, Suzuki K, Nakayama H. Immunohistochemical Characterization of Canine Neuroepithelial Tumors.
Vet Pathol. 2010;47(4)741-750.
3. Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ. Oligodendroglioma. In:
Histological Classification of Tumors of the Nervous System of Domestic Animals. 2nd series. Vol. V. Washington, DC: Armed Forces Institute of Pathology; 1999:19-20.
4. Summers BA, Cummings JF, DeLahunta A. Oligodendroglial tumors. In:
Veterinary Neuropathology. St. Louis, MO: Mosby-Year Book Inc; 1995:370-373.
5. Tomek A, Cizinauskas S, Doherr M, Gandini G, Jaggy A. Intracranial neoplasia in 61 cats: localisation, tumour types and seizure patterns.
J Feline Med Surg. 2006;8(4):243-53.
6. Troxel MT, Vite CH, Van Winkle TJ, Newton AL, Tiches D, Dayrell-Hart B, et al. Feline intracranial neoplasia: retrospective review of 160 cases (1985-2001).
J Vet Intern Med. 2003;17(6):850-9.
7. Zachary JF. Nervous system. In: McGavin MD, Zachary JF, eds.
Pathologic Basis of Veterinary Disease. 4th ed., St Louis, MO: Mosby Elsevier; 2007:951.