Joint Pathology Center
Veterinary Pathology Services
Wednesday Slide Conference
2019-2020
Conference 13
8 January 2020
CASE I: 2019A208 Case 2 (JPC 4130570).
Signalment: 4 months, female, Rhodesian Ridgeback, Canis familiaris, dog
History: The dog was presented on emergency for acute lethargy, anorexia and melena. Blood examination revealed increased CK and liver enzymes. On echography and clinical examination generalized lymphadenopathy was noticed. Soon the dog developed neurological signs with decreased consciousness, compulsive behavior and head pressing (forebrain disorder.) Also coagulopathy due to severe thrombocytopenia with increased coagulation times (PT and aPTT) was present. In 24 hours there was a progression of symptoms with neurological deterioration. Finally cardiac arrest developed with unsuccessful reanimation; the clinicians postulated hemorrhagic disorder, infectious or immune mediated encephalitis and metabolic encephalopathy as main differential diagnosis. Vaccination and deworming was up to date.
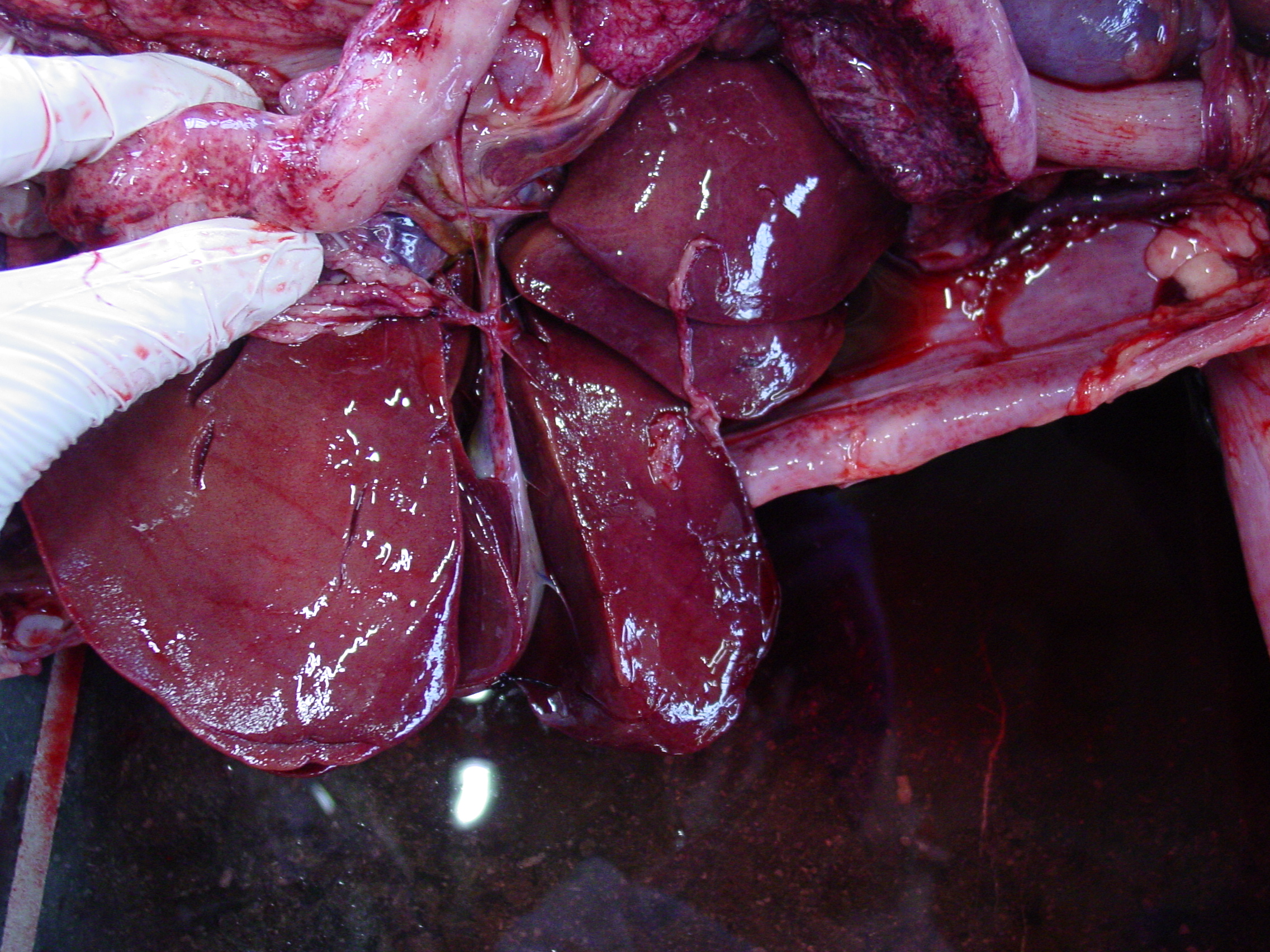
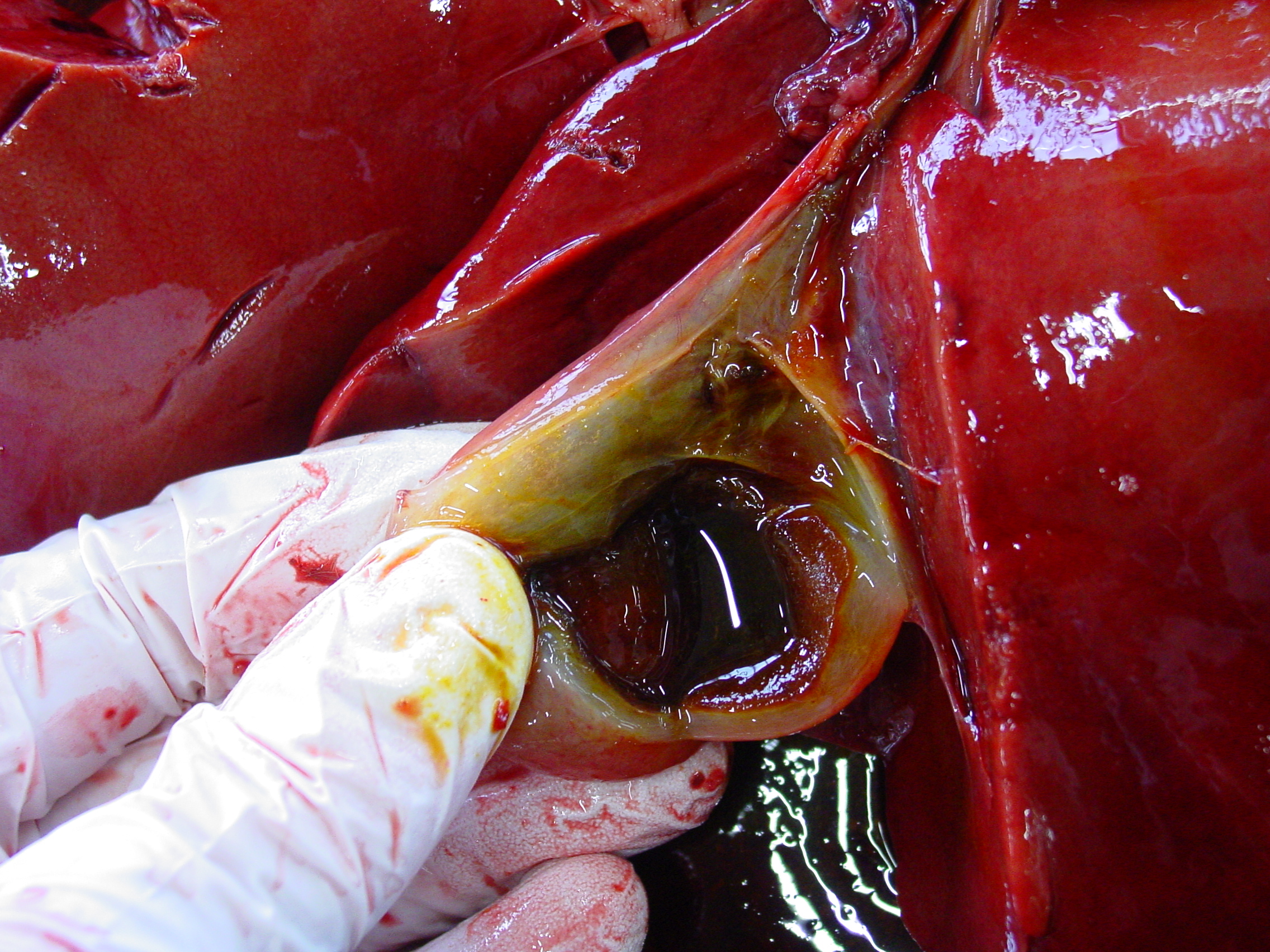
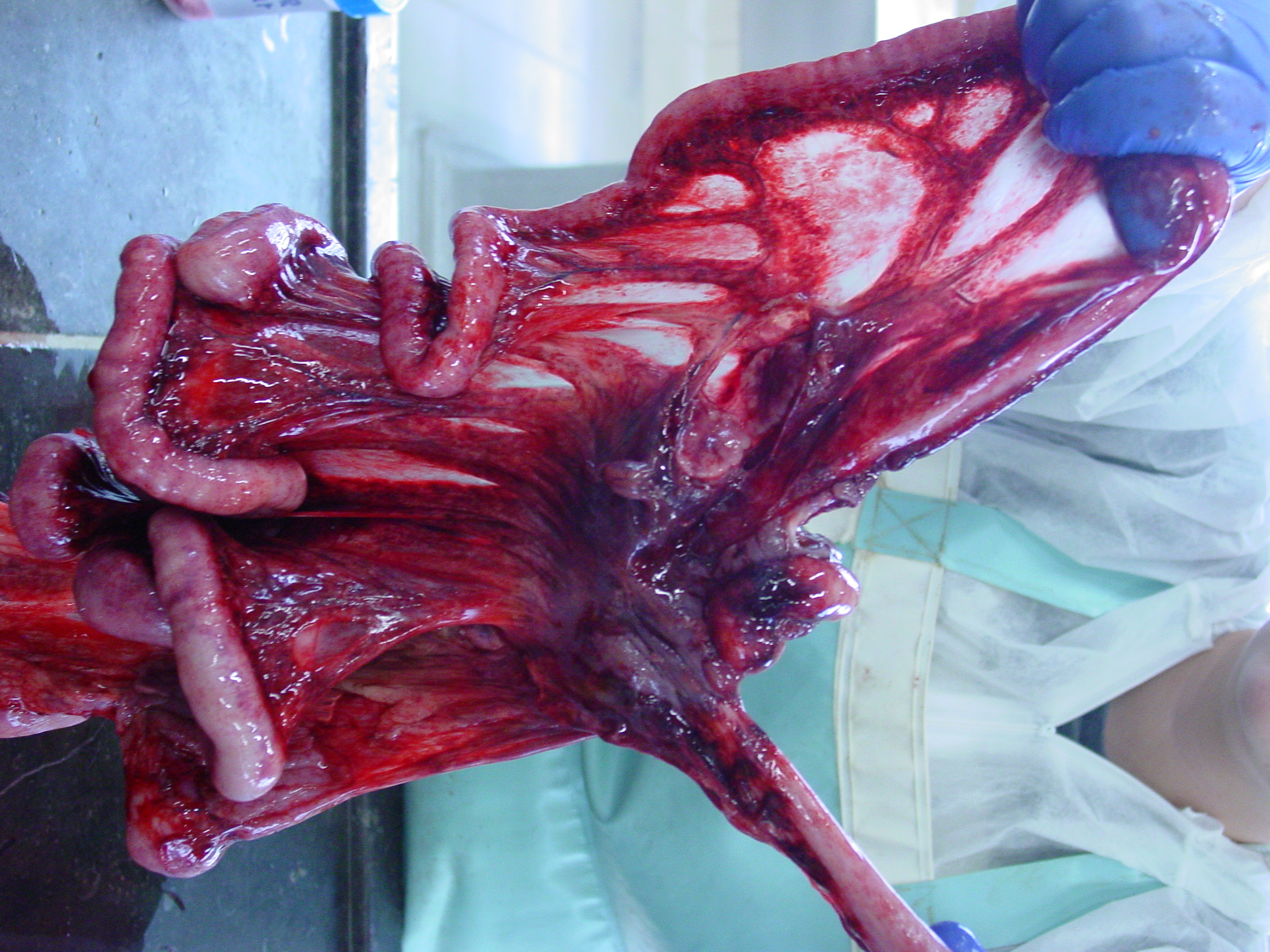
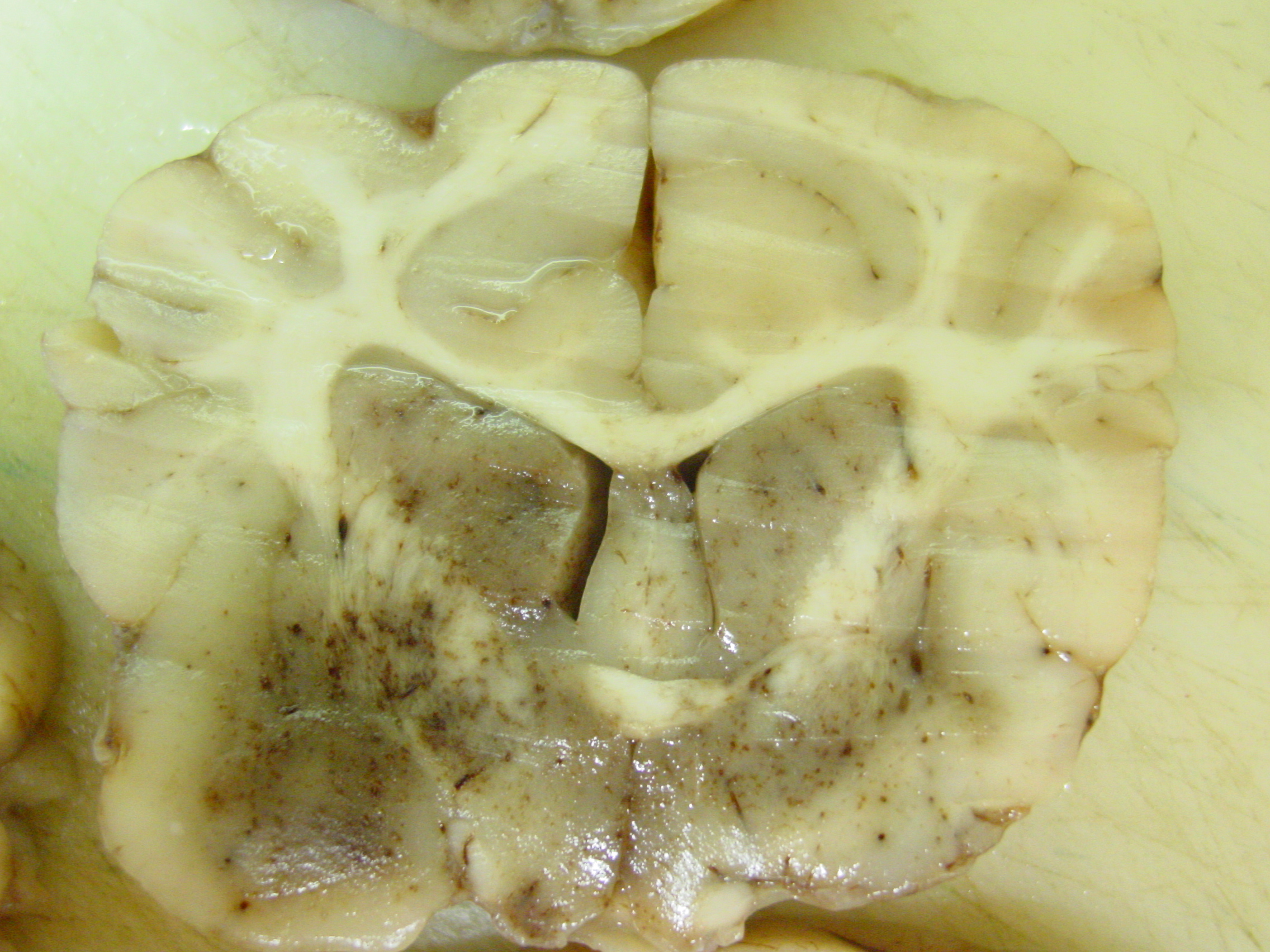
Gross Pathology: The liver was moderately pale, enlarged with a scant amount of fibrin strands on the capsule. There were several ruptures secondary to reanimation causing hemoabdomen. Spleen and lymph nodes were severely enlarged. The mesenteric lymph nodes were clearly edematous on cut surface. Many hemorrhages were seen at the serosal surfaces. Also severe edema of the gallbladder and melena was present. Dispersed small hemorrhages were present in the basal nuclei of the cerebrum and in the thalamus of the brain on cut surface.
Laboratory results:
- Blood examination: increased CK 1199U/L (99-436), increased GOT/GPT and bile acids (no value available), thrombocytopenia 23 K/μL (148-484) and increased PT 18s (11-17) and aPTT 144s (72-102).
-Toxoplasmosis and neosporosis were excluded (IgG/M-determination.)
- Parvovirosis and angiostrongylosis were tested with a negative result.
Microscopic Description:
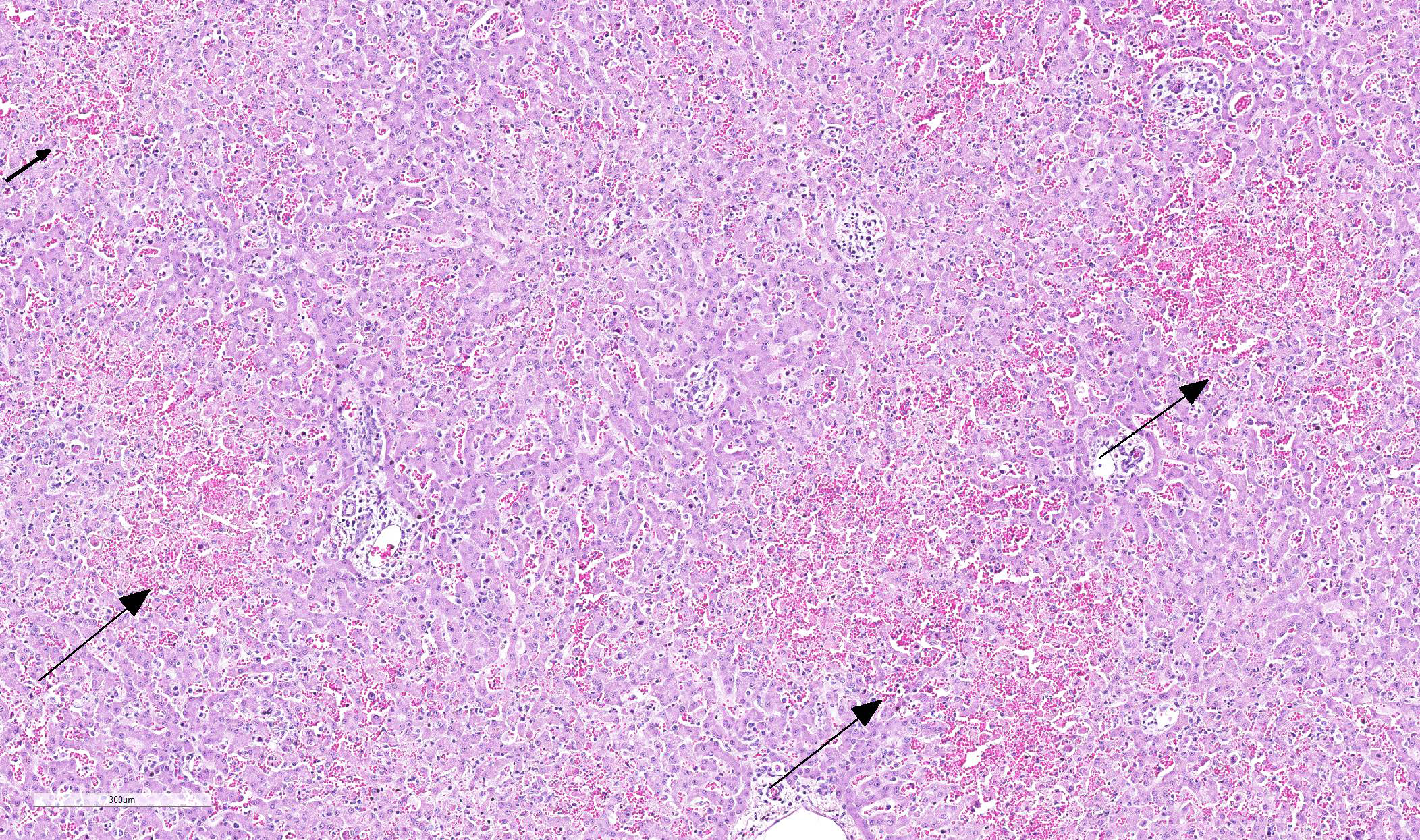
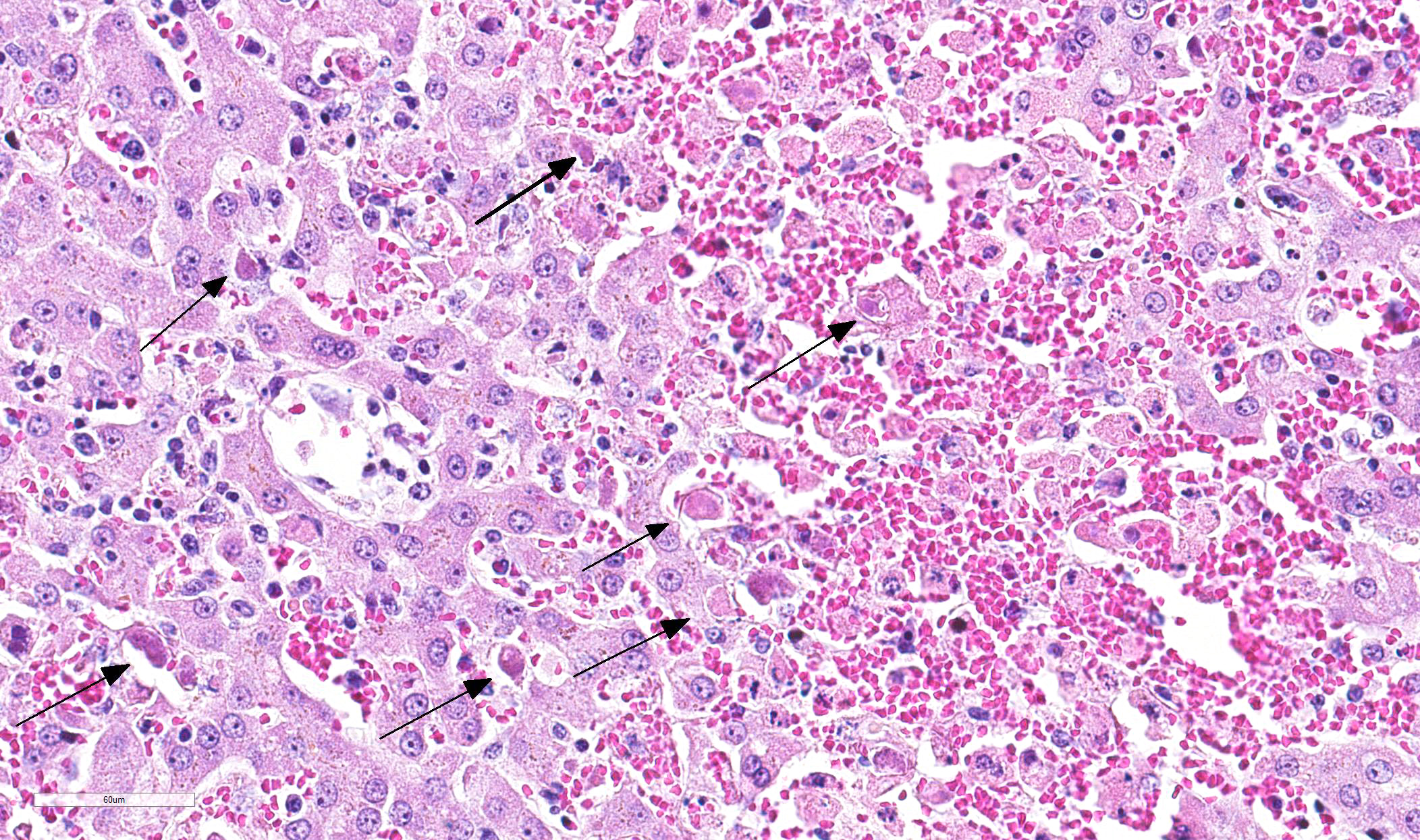
Multifocal in the midzonal to centrilobular region, areas of coagulation necrosis are present. This is characterized by swollen, eosinophilic, well circumscribed hepatocytes with a dark small, fragmented nucleus. In these areas loss of tissue architecture is present with pooling of red blood cells. Multifocal distributed, several hepatocytes and Kupffer?s cells contain large, intranuclear basophilic inclusions surrounded by a clear halo with margination of chromatin. In the sinusoidal spaces, there is a moderate increased amount of lymphocytes. There is a large subcapsular bleeding (secondary to liver rupture due to cardiac reanimation). On immunohistochemical staining for Canine adenovirus, there are numerous hepatocytes and Kupffer?s cells with positive staining nuclei.
Contributor Morphologic Diagnosis:
Severe multifocal midzonal to centrilobular hepatic necrosis with large basophilic intranuclear viral inclusion bodies, consistent with canine adenovirus type 1.
Contributor Comment: Non-enveloped dsDNA virus à family: Adenoviridae à genus: Mastadenovirus ; there are 2 types.10
- Canine adenovirus - 1 (CAV-1) causes infection in numerous mammalian carnivores belonging to the Canidae, Mustelidae and Urisdae. In dogs, it causes infectious canine hepatitis (ICH) also called fox encephalitis or Rubarth?s disease.9 This virus causes a systemic infection mainly targeting the hepatocytes, endothelial and mesothelial cells. CAV-1 can cause a severe often fatal disease in juveniles. Death is very rare in animals older than 2 years of age. Clinical symptoms are variable, often vomiting, melena, fever and abdominal pain is present. In some cases nonspecific nervous signs occur. In a minority of cases there is acute death without preceding symptoms. During the recovery phase uni- or bilateral corneal edema can develop (?blue eye?).5 Years of widespread vaccination reduced the incidence of this disease in domestic animals in many countries to almost zero. In wildlife species CAV-1 is widespread, primarily as a subclinical infection but epizootic disease occurs1. In foxes neurological symptoms are more frequently present than in dogs being the reason for its less popular name: fox encephalitis virus.2
- CAV-2 causes a mild self-limiting infection of the upper respiratory tract and plays a role in the pathogenesis of infectious tracheobronchitis (ITB) known as kennel cough. Because of antibody cross-reaction it is used for CAV-1 vaccine production.2
Pathogenesis:
Infection occurs through oronasal transmission. After reaching the oropharyngeal tonsils, tonsillitis develops accompanied by fever. The virus spreads to local lymph nodes and reaches the bloodstream through the lymph flow causing viremia which lasts 4-8 days. In a following phase the primary targets: hepatic parenchymal cells and endothelial cells (in al organs) get infected4. Viral replication causes cell lysis and necrosis.13 Hepatic necrosis ensues at about day 7 (of experimental infection).2 Lesions in other organs are mainly secondary to vascular injury causing hemorrhage and edema. In a further stage, disseminated intravascular coagulation (DIC) can occur.10 This is caused by lysis of endothelial cells with activation of coagulation cascade and aggregation of thrombocytes. This causes increased consumption of clotting factors and thrombocytes resulting in consumptive coagulopathy.11 Several organ systems can be affected including the liver, kidney, lymph nodes, thymus, gastric serosa, pancreas and subcutaneous tissues.12 Edema of the gallbladder can be very prominent and is most likely due to increased vascular permeability secondary tot vascular injury. Gallbladder edema is a pathognomonic sign at necropsy.7 The central nervous system can be also be targeted causing an encephalitis with often inconspicuous lesions.
During the convalescent phase (day 7-21 days post infection) deposition of immune complexes (antigen-antibody) in the cornea can occur causing complement fixation and neutrophilic activation (type III hypersensitivity). The neutrophilic proteases cause diffuse hydropic degeneration of corneal endothelium and secondary stromal edema. The interstitial fluid accumulation causes deformation of corneal collagen fibers creating a blue-white aspect of the cornea (?blue eye?). The same mechanism happens in a small percentage of dogs 6-7 days after vaccination with modified live virus. In some cases inflammation proceeds and causes interstitial keratitis and permanent fibrosis. Alongside the corneal edema, anterior uveitis and glomerulonephritis can develop because of type III hypersensitivity.4,8
Gross findings:
- Hepatomegaly; turgid and friable with sometimes a mottled yellowish aspect, fibrin strands at the capsular surface6
- Edema and intramural bleeding of the gallbladder (pathognomonic)7
- Enlarged, hemorrhagic, edematous lymph nodes
- Serosal bleeding (paintbrush lesions)
Gross lesions in other organs are inconstant:5
- Hemorrhagic kidney infarctions (in young puppies)
- Enlarged, edematous tonsils (tonsillitis)
- Hemorrhages in the lungs, brain (midbrain and brainstem) and metaphyses of the long bones (ribs).
- Multifocal mucosal petechiae and subserosal hemorrhage11
- Corneal edema (?blue eye?)
Histological findings:
Liver:
- Centrilobular (periacinar) zonal necrosis (resembling acute hepatotoxicity.) In convalescence, hepatic regeneration occurs rapidly. After 2 weeks small foci of hepatocellular necrosis may be present. Foci of regenerating Kupffer?s cells may be present for another 2 weeks6
- Intranuclear, large, basophilic inclusion bodies in endothelial cells, hepatocytes or Kupffer?s cells. Viral inclusions can be present after 4 days of experimental infection. At day 5-6-7 inclusions are most numerous, from day 8 numbers decline rapidly (in experimental infection)2
- Fatty change
- Blood filled dilated sinusoids (because of loss of hepatocytes)
- Intact reticulin framework
- Mild leukocytic infiltration, mostly degenerating neutrophils
Other organs: Microscopic lesions in other organs are mainly secondary to endothelial damage.
- Brain: Hemorrhages of small vessels with rare intranuclear inclusions in endothelial cells which can be in relation with small foci of demyelination. Encephalitis can develop with very subtle lesions characterized by a mononuclear vasculitis with rarely more than a single perivascular monolayer of cells and hemorrhage9
- Lymphoreticular organs: Congestion, lymphocytolysis in lymphoid follicles and in the white pulp of the spleen,9 inclusions can be present in reticulum cells
- Kidney: Occasional intranuclear inclusion body in the glomerular mesangium cells or in the epithelial cells of the proximal convoluted tubule.9. Glomerulonephritis and secondary chronic interstitial nephritis can develop because of deposition of immune complexes in later stages.1,4
- Lung: areas of hemorrhagic consolidation with hemorrhage, edema and fibrin formation in the alveoli with often inclusions in alveolar capillaries.
- Eye: corneal edema due to hydropic degeneration of corneal endothelium which can develop into interstitial keratitis and permanent fibrosis. Intranuclear inclusions can be present in a few degenerating endothelial cells. There can be accompanying anterior uveitis characterized by accumulation of lymphocytes and plasma cells perivascular in the iris and ciliary body.8
- In every organ macrophages with intranuclear inclusions can be present.5
Contributing Institution:
University of Ghent
https://www.ugent.be/di/di05/nl
JPC Diagnosis: Liver: Hepatitis, necrotizing, multifocal, mild to moderate, with edema and numerous eosinophilic hepatocellular and endothelial intranuclear viral inclusion bodies.
JPC
Comment: The contributor
has given us an excellent and very comprehensive review of canine adenovirus-1
in the dog.
Liver infection with adenovirus have been a popular, although not especially
diagnostically challenging, submission over the years in a number of species.
This case marks the seventh time that CAV-1 has appeared in the WSC in the last
forty years. Adenovirus in the liver of chickens (inclusion body hepatitis)
has appeared a total of six times, with falcon adenovirus and bearded dragon
(agamid) adenovirus appearing twice. (The complete list of adenoviral submittions
over the years is well over a hundred, with pulmonary infections being the most
common overall.)
In 1998, a case of CAV-1 in a skunk which manifested as fatal necrotizing hepatitis was reveiwed in conference number 4, suggesting that this virus may cause disease in other wildlife species outside of canids. Although the conference results incorrectly identify skunks as canids (they are mustelids!), foxes, wolves and coyotes have certainly developed fatal disease associated with CAV-1. Skunks indeed have their own adenovirus (skunk adenovirus-1, first identified in 20155) which also causes fatal hepatitis (casting a bit of doubt over our 1998 case of CAV in a mustelid.) While adenoviruses are generally considered species-specific, skunk adenovirus-1 has been identified as causing pneumonia and trachieitis in pygmy hedehogs as well.6 Several species of adenoviruses have also been identified by nested PCR in mustelids in the United Kingdom, but associated disease has not been ascribed to these viruses.11
The most spirited discussion on this very classic case regarded the location of necrosis. While textbooks often discuss a characteristic pattern of centrilobular necrosis in affected animals, the typical pattern for viral hepatic infection would be expected to be random. Additionally, within many areas of necrosis, effete hepatocytes contain a basophilic stippling which some participants questioned might be an apicomplexan cyst. However, the material stained stongly positive with a Prussian Blue stain indicated that the material was iron-based. The cause of the ferrugination of occasional degenerate/necrotic cells was not clear.
References:
1. Balboni A, Verin R, Morandi F, et al. Molecular epidemiology of canine adenovirus 1 and type 2 in free-ranging red foxes (Vulpes vulpes) in Italy. Veterinary Microbiology. 2013; (162): 551-557.
2. Cabasso VJ. Infectious canine hepatitis virus. Annals of the New York Academy of Sciences. 1962; (101): 498-514.
3. Cabasso VJ. Infectious canine hepatitis. In: Infectious Diseases of Wild Animals, Davis, Karstad, Trainer eds., Ames: Iowas State University Press, Ames, Iowa, 1981.
4. Cianciolo RE, Mohr FC. Urinary System. In: Maxie MG, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Vol. 2. 6th ed. St. Louis MO: Elsevier Ltd; 2016:410.
5. Lozak RA, Ackford JG, Slaine P, LiA, Carman S, Campbell D, Welch MK, Kropinskin AM, Nagy, E. Characterization of a novel adenovirus isolated from a skunk. Virology 2015; 485:16-24.
6. Needle DB, Selig M, Jackson KA, Delwart E, Tighe E, Leib SL, Seuberlich T, Pesavento PA. Fatal bronchopneumonia caused by skunk adenovirus 1 in an African pygmy hedgehog.
7. Stalker MJ, Hayes MA. Liver and biliary system. In: Maxie MG, ed. Jubb, Kennedy and Palmer?s Pathology of Domestic Animals. 5th ed. Vol 2. New York, NY: Elsevier Saunders; 2007:348-351.
8. Vandevelde M, Higgins RJ, Oevermann A. Inflammatory diseases. In: Veterinary Neuropathology: Essentials of Theory and Practice. 1th ed. West Sussex, UK: John Wiley & Sons Ltd; 60-61.
9. Wilcock BP, Njaa BL. Special Senses. In: Maxie MG, ed. Jubb, Kennedy, and Palmer?s Pathology of Domestic Animals. Vol. 1. 6th ed. St. Louis MO: Elsevier Ltd; 2016:452-453.
10. Walker D, Abbondati E, Cox AL, et al. Infectious canine hepatitis in red foxes (Vulpes vulpes) in wildlife rescue centres in the UK. Veterinary Record. 2016; (178) : 421.
11. Walker D, Gregory WF, Turnbull D, Rocchi M, Meredith AL, Philbey AW, Sharp CP. Novel adenoviruses detected in British mustelids, including a unique aviadenovirus in the tissues of pine martens. J Med Microbiol 2017; 66:1177-1182.
12. Wong M, Woolford L, Hasan NH, Hemmatzadeh F. A Novel Recombinant Canine Adenovirus Type 1 Detected from 2 Acute Lethal Cases of Infectious Canine Hepatitis. Viral Immunology. 2017; (30): 258-263.
13. Wigton DH, Kociba GJ, Hoover EA. Infectious Canine Hepatitis: Animal Model for Viral-Induced Disseminated Intravascular Coagulation. Blood. 1976; (47): 287-296.
14. Zachary JF. Mechanisms of microbial infections. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 2017:207.