Wednesday Slide Conference, Conference 19, Case 4
Signalment:
Adult, male, blue and white budgerigar (Melopsittacus undulatus)
History:
This adult male budgerigar was housed in an indoor aviary. He had been vaccinated against West Nile virus in May, 2020. Spring of 2021, he was found dead with no reported antemortem clinical signs.
Gross Pathology:
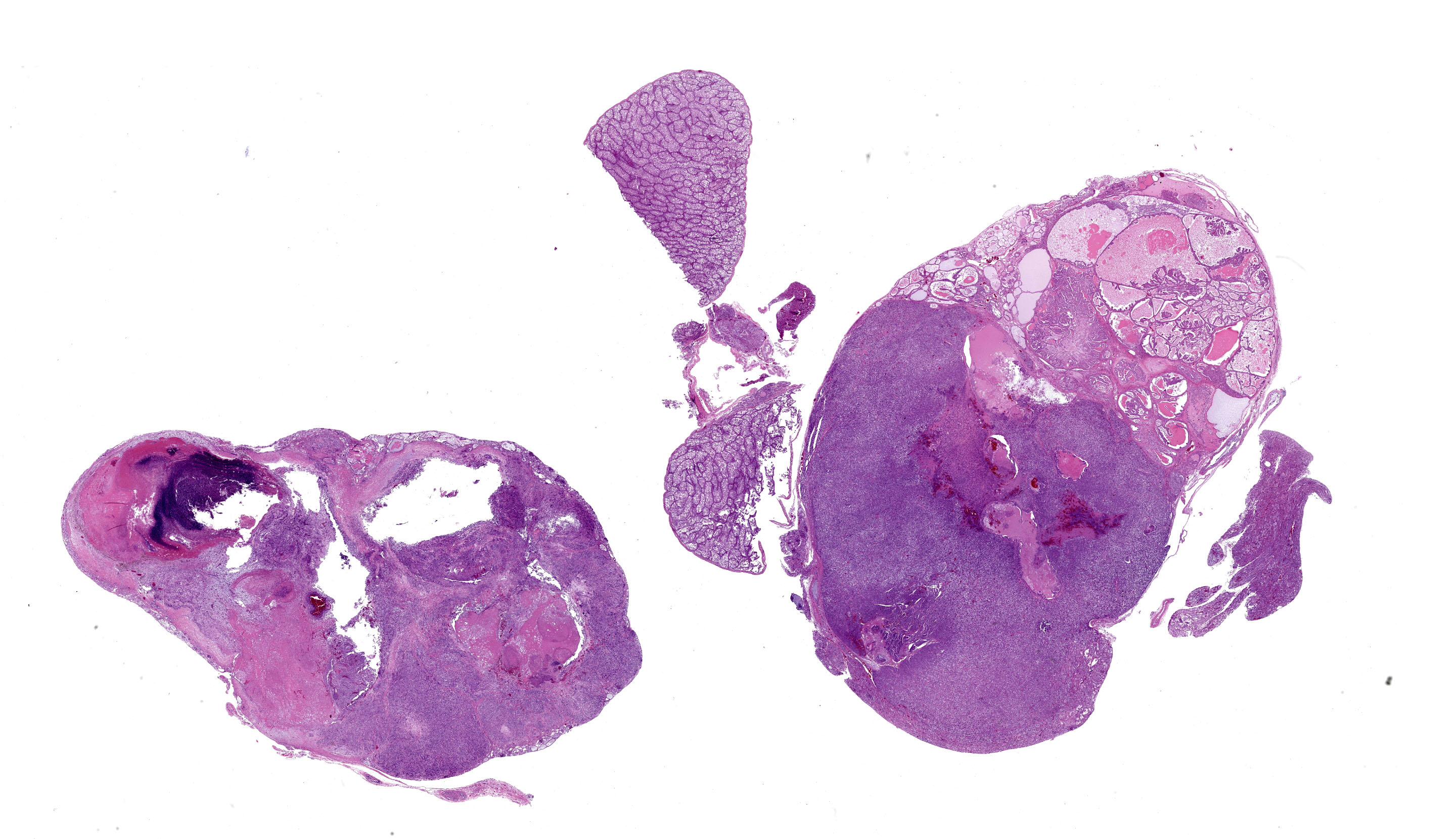
Bilaterally effacing the kidneys is a multilobulated, poorly circumscribed, tan, soft mass. The mass moderately displaces the testes, cranially. There are scant subcutaneous and visceral adipose stores.
Laboratory Results:
Chlamydia psittaci, avian polyomavirus, and psittacine herpesvirus were not detected by real time PCR on pooled lung, liver, and kidney samples.
Microscopic Description:
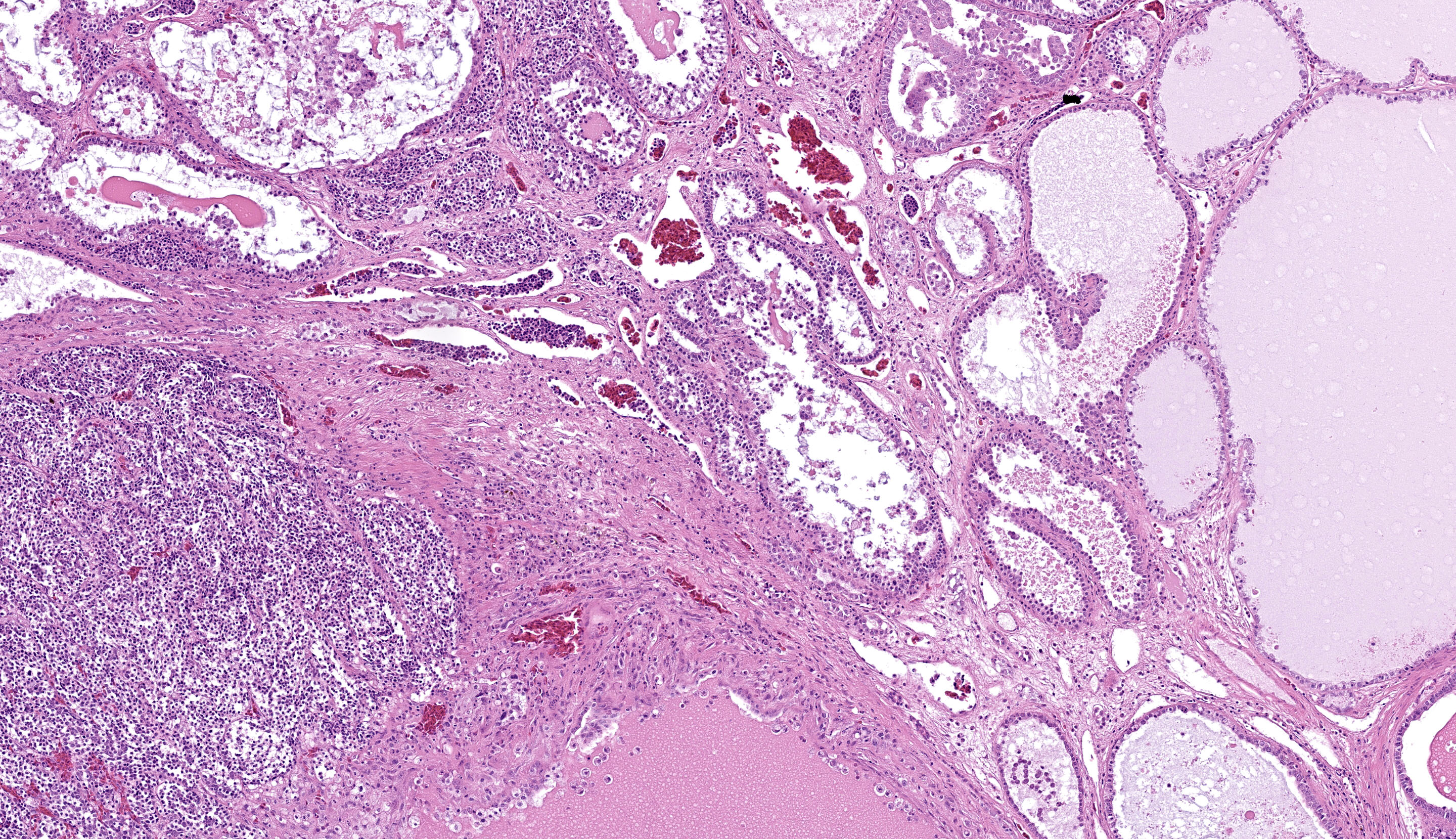
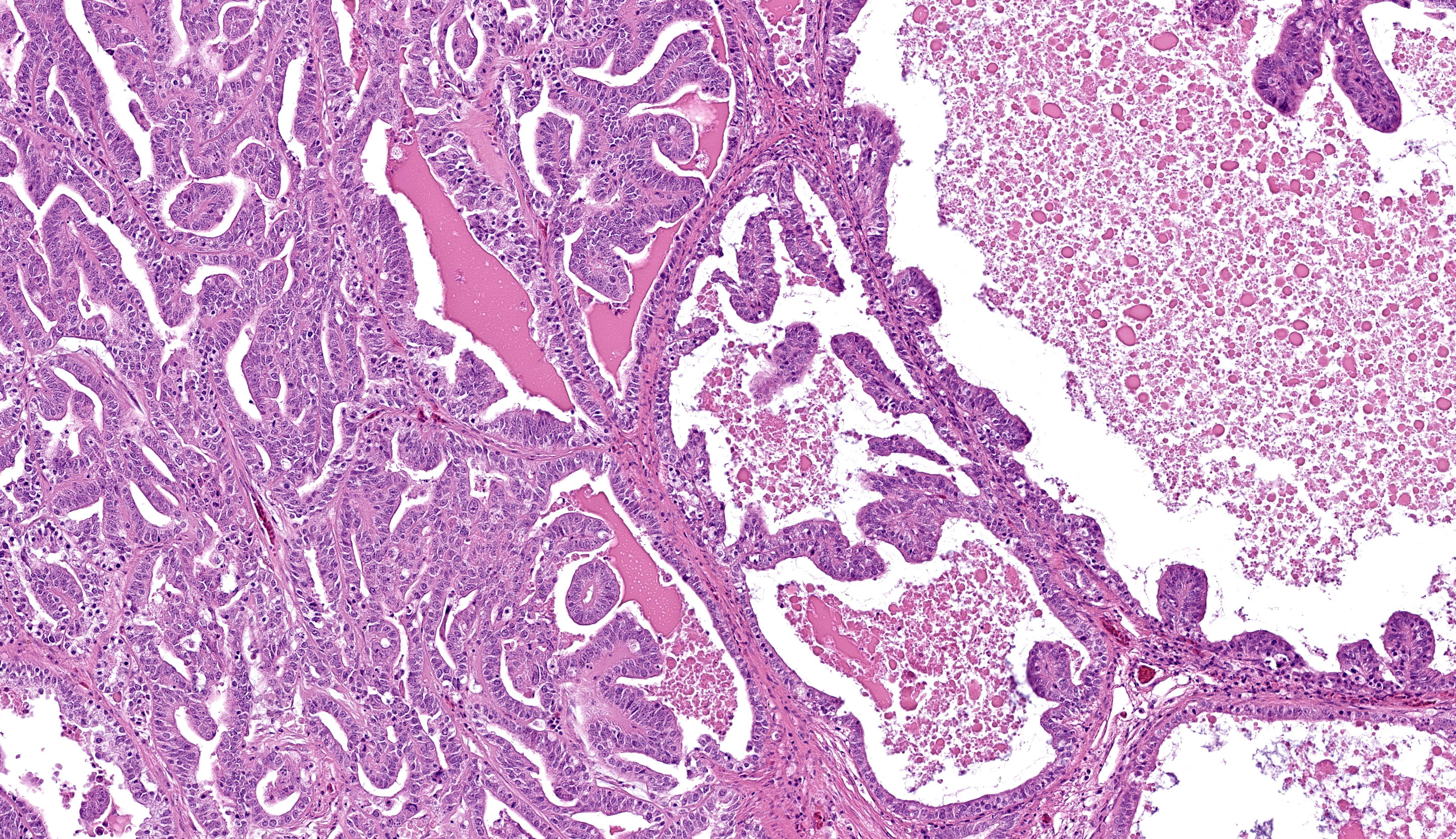
Kidney, left and right: Expanding and effacing both examined sections of kidney is a large, multinodular, unencapsulated, densely cellular infiltrative neoplasm. The polygonal neoplastic cells variably form haphazardly organized tubules, papillary proliferations, and occasional cords arranged over a fine fibrovascular stroma. The neoplastic epithelial cells have distinct cell borders, scant to moderate amounts of clear to granular eosinophilic cytoplasm, and a single, basally to centrally located round nucleus with finely stippled chromatin and one variably distinct amphophilic to eosinophilic nucleolus. There is mild to moderate anisocytosis and anisokaryosis. There is approximately one mitotic figure per high power (400x) field. There are large multifocal regions of necrosis and hemorrhage with hemosiderin laden macrophages and heterophils scattered throughout the neoplasm. Mild to moderate amounts of bright homogenous eosinophilic material (proteinaceous fluid) occasionally fills neoplastic and remaining normal renal tubular lumina. The remaining normal renal parenchyma is compressed at the periphery of the mass. The remaining pre-existing renal tubules occasionally exhibit one or more of the following changes: mild to moderate ectasia, epithelial attenuation, pale vacuolated eosinophilic cytoplasm with loss of cellular detail (degeneration), and/or colorless to basophilic radiating, sharp, acicular, crystalline deposits (urate tophi), often accompanied and surrounded by heterophils and macrophages.
Contributor’s Morphologic Diagnosis:
Kidney: Renal adenocarcinoma, budgerigar
Contributor’s Comment:
The cellular morphology and arrangement of the mass in this case are characteristic of renal tubular epithelial origin.
Renal carcinomas and adenomas are significantly more common in budgerigars than other psittacine species, although the mechanisms for this are not completely understood.6 Captive birds are generally understood to have a higher incidence of neoplasia than wild birds, potentially due to differences in life span, diet, and carcinogen exposure.8 Several carcinogens, including but not limited to aflatoxins, lead, and nitrosamines, are thought to contribute to renal carcinoma development in veterinary species.4 In addition to carcinogen exposure, several viral etiologies have been identified in renal carcinomas in some species, including induction of renal adenocarcinoma via avian erythroblastosis virus in chickens1 and renal adenocarcinomas, also known as Lucké’s tumor of leopard frogs, by ranid herpesvirus type 1 (RaHV-1).3 Investigations into potential retroviral causes of renal neoplasms in budgerigars have not identified associated retroviruses.7
The most frequent presenting clinical sign of renal adenocarcinoma in birds is unilateral lameness due compression of the sciatic nerve.8 The gross appearance of the renal adenocarcinoma in this case was very characteristic. Most renal adenocarcinomas are large, tan to reddish brown, soft to friable, and are located at the cranial pole of the kidneys (in this case, displacing the testes cranially).8 Histologically, the variation in arrangement of tubules, papillary projections and more solid areas of tightly packed cords confirm the suspected diagnosis of renal cell adenocarcinoma. Renal adenomas have less cellular pleomorphism and are comprised of tubules lined by a single layer of epithelium with a moderate fibrovascular stroma. Avian renal carcinomas and adenocarcinomas are reported to infrequently metastasize to the lungs and liver.6
A majority of the immunohistochemical characterization of renal adenocarcinomas and renal cell carcinomas has been done in canine patients. Immunohistochemistry for uromodulin and uroplakin may be useful for confirming tissue of renal origin, as these markers are commonly expressed by canine renal tumors.2 Additionally, canine renal cell carcinomas frequently co-express cytokeratin and vimentin, and express CD10, PAX 8, and napsin A.5 Though no immunohistochemistry was pursued in this case, it may be useful to try the above-mentioned markers in renal tumors from budgies if the diagnosis based only on morphology is in question.
In contrast to budgerigars, primary renal neoplasms are uncommon in most domestic veterinary species. The most common primary renal neoplasm in dogs, cats, cattle, and horses are renal cell carcinomas rather than adenocarcinomas. Additional differential diagnoses for a primary renal mass include renal adenoma, oncocytoma, and transitional cell papilloma and carcinoma. Renal adenomas are rare, benign epithelial neoplasms which are not commonly reported in budgerigars.1
Contributing Institution:
University of Wisconsin
School of Veterinary Medicine
Department of Pathobiological Sciences
2015 Linden Drive
Madison, WI 53706
JPC Diagnosis:
Kidney: Renal adenocarcinoma
JPC Comment:
The final case of this conference is another classic neoplasm, this time in a budgie. The contributor gave us several options among slides for this case. The digital section that we ultimately chose has a great assortment of ancillary features, though it might initially be misleading on tissue identification with the normal testis distracting from the relatively small portion of remaining renal cortex to evaluate. We agree that this neoplasm is a renal adenocarcinoma that has both solid and papillary-predominant regions mixed with necrotic foci. In solid regions, neoplastic cells have increased cytoplasmic clearing which has been described as a clear cell histologic subtype.
We took the contributor up on their challenge to try canine renal cell carcinoma markers for this case. Napsin A, CD10, CK7, vimentin, and uroplakin were all immunonegative. We noted moderate-to-strong nuclear immunoreactivity to PAX8 within 30% of neoplastic cells (predominately in well-differentiated cells in the papillary-predominant region), consistent with renal epithelial cell origin. This distribution also aligns with PAX8 performance in canine RCCs.5 The remaining renal tubules served as a useful internal tissue control. That stated, there is conflicting information that the avian genome (or at least chickens) lack an orthologous Pax8 gene9 despite this convincing antibody performance. Polyclonal PAX8 antibodies do cross label with PAX5 – review of lab materials indicate that we used a monoclonal mouse primary antibody (MRQ-50; Ventana) which removes this possibility. We look forward to trying this antibody with appropriate controls in future cases (see discussion in case 2 of this conference) should the opportunity arise.
References:
- Breshears MA, Confer AW. The Urinary System. In: Zachary JF ed. Pathologic Basis of Veterinary Disease. 6th ed. Elsevier; 2017.
- Cianciolo RE, Mohr FC. Urinary System. In: Maxie MG ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. Elsevier; 2016.
- McKinnel RG, Carlson DL. Lucke renal adenocarcinoma, an anuran neoplasm: Studies at the interface of pathology, virology, and differentiation competence. J Cell Physiol. 1997;173:115-118.
- Meuten DJ, Meuten TLK. Tumors of the Urinary System. In: Meuten DJ ed.Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Inc; 2017.
- Peat TJ, Edmondson EF, Miller MA, DuSold DM, Ramos-Vara JA. Pax8, Napsin A, and CD10 as immunohistochemical markers of canine renal cell carcinoma. Vet Pathol. 2017;54:588-594.
- Schmidt RE, Reavill DR, Phalen DN. Pathology of Pet and Aviary Birds. Iowa State Press; 2003.
- Simova-Curd SA, Huder JB, Boeni J, Robert N, Hatt JM. Investigations on the diagnosis and retroviral aetiology of renal neoplasia in budgerigars (Melopsittacus undulatus). Avian Pathol. 2010;39:161-167.
- Simova-Curd S, Nitzl D, Mayer J, Hatt JM. Clinical approach to renal neoplasia in budgerigars (Melopsittacus undulatus). J Small Anim Pract. 2006;47:504-511.
- Freter S, Muta Y, O'Neill P, Vassilev VS, Kuraku S, Ladher RK. Pax2 modulates proliferation during specification of the otic and epibranchial placodes. Dev Dyn. 2012 Nov;241(11):1716-28.