Signalment:
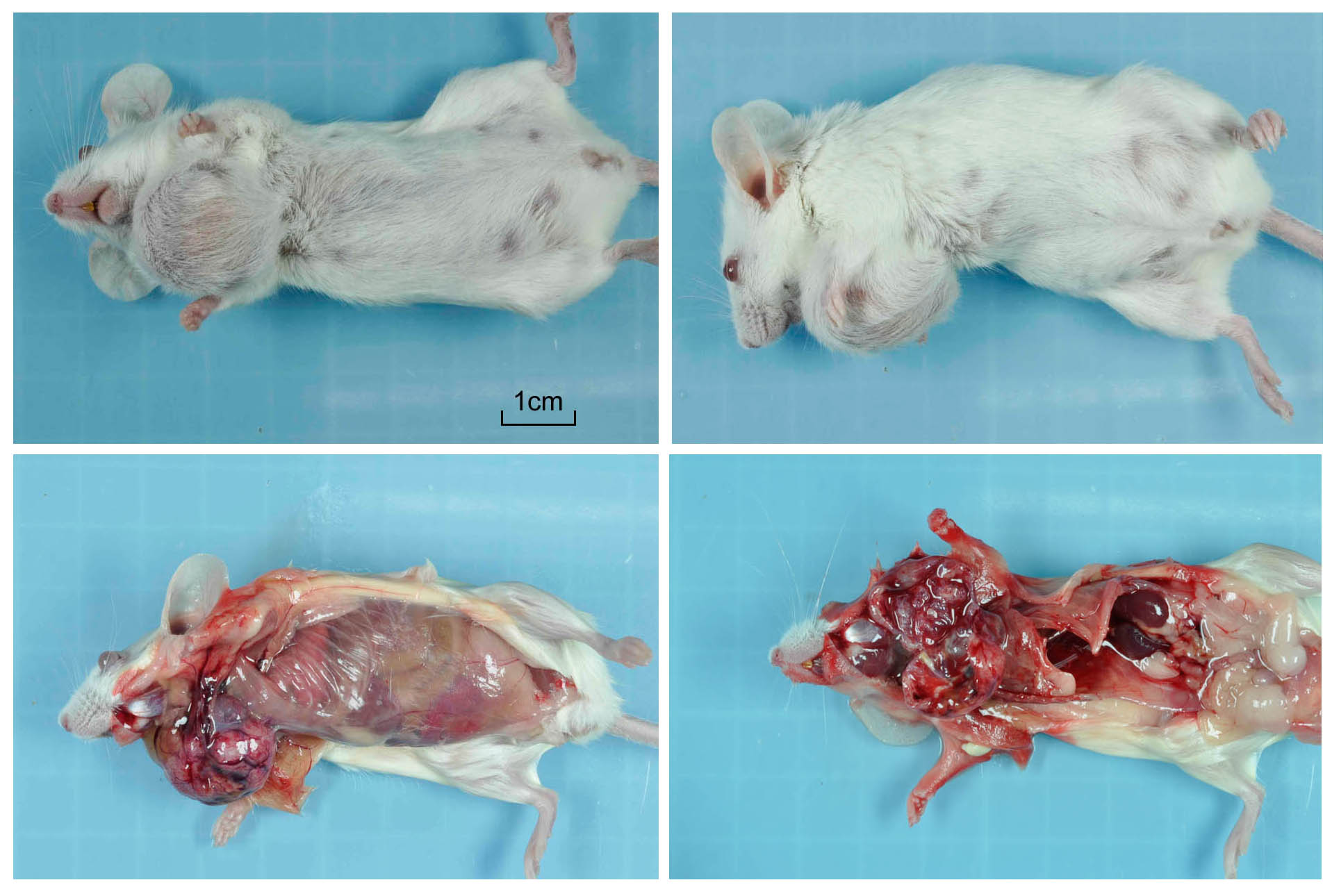
Gross Description:
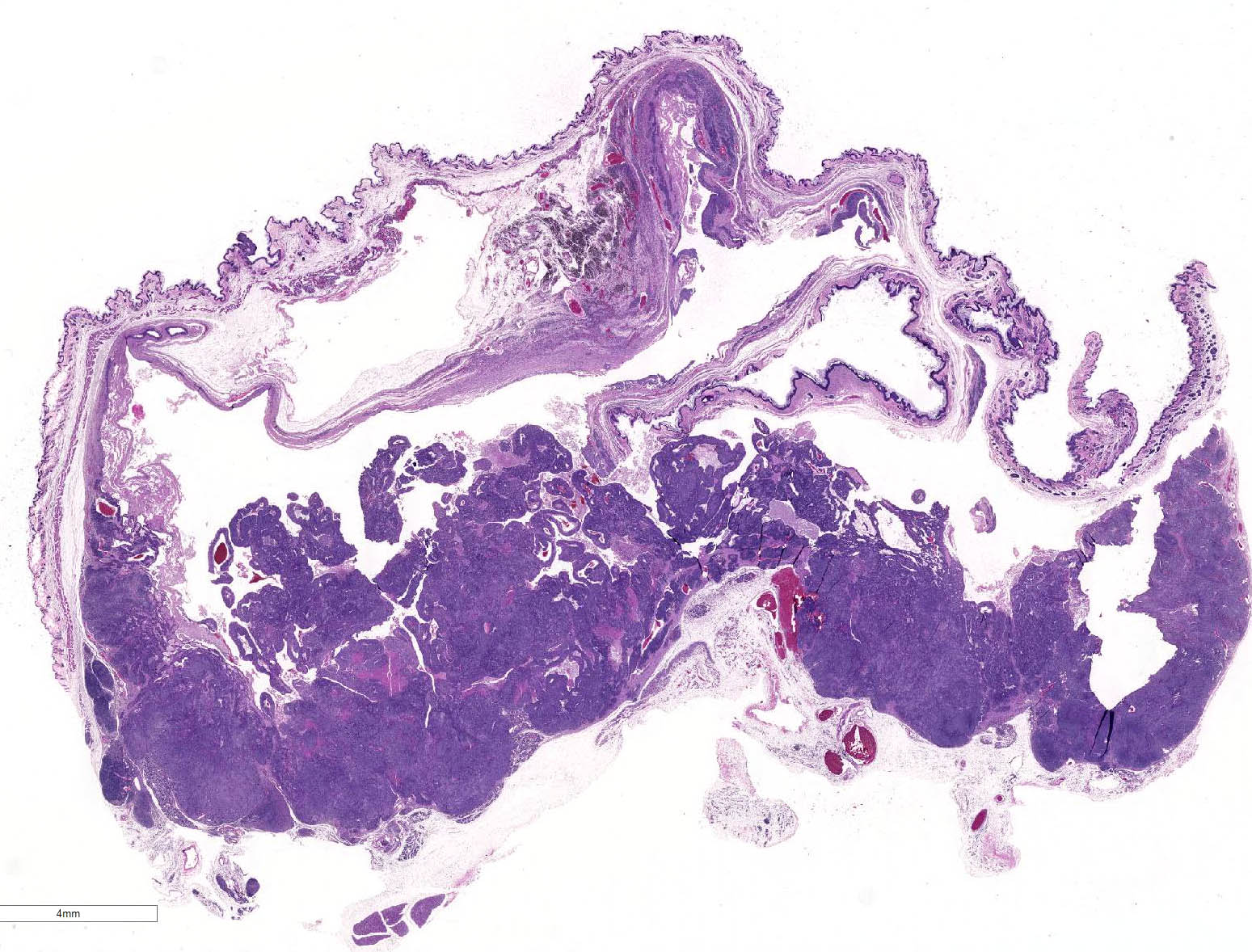
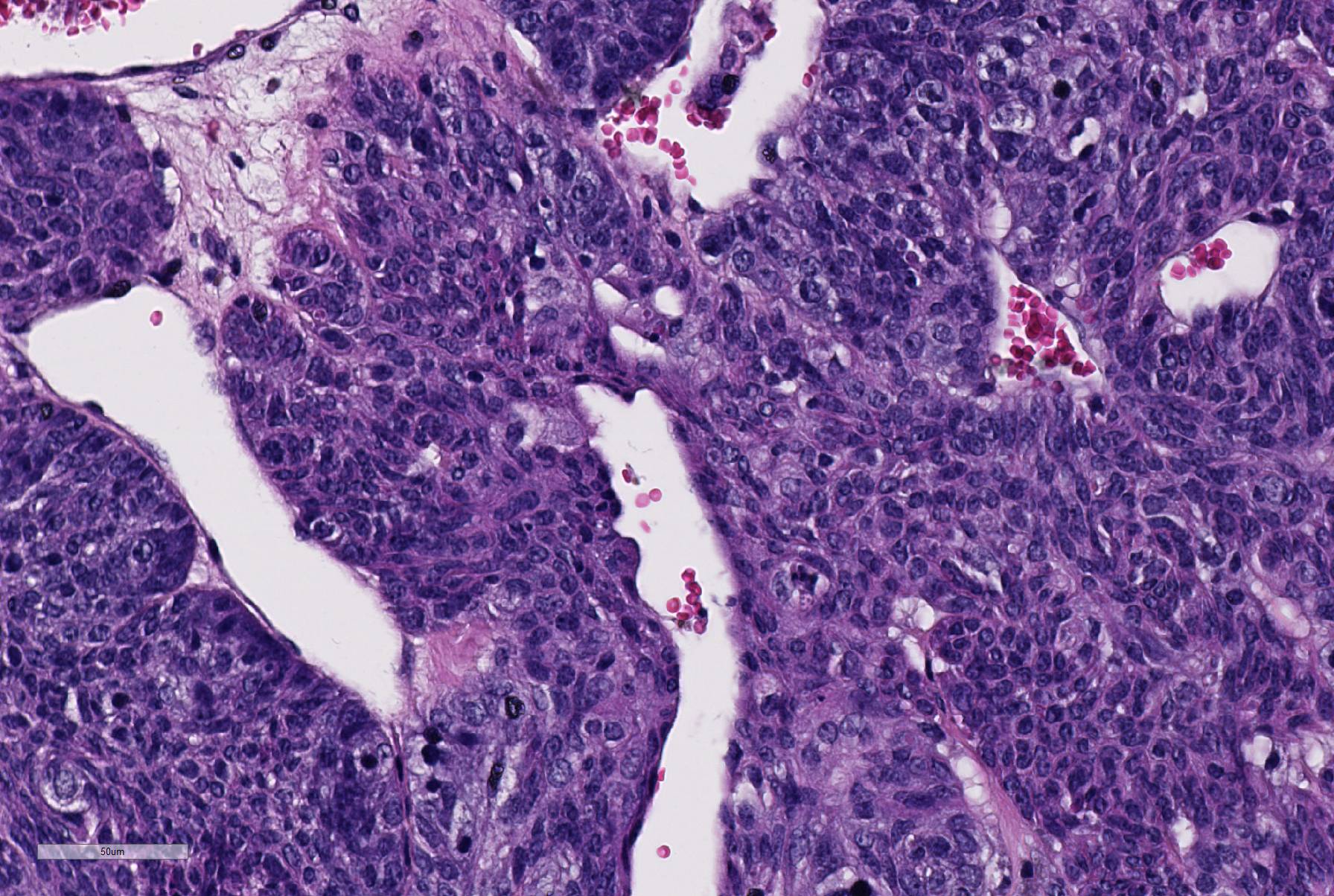
Histopathologic Description:
There is a well-circumscribed multinodular and cystic subcutaneous mass composed of disorganized proliferation of plump spindle to polygonal cells arranged into interlacing fascicles, solid lobules and thick bands where the cells are often arranged in a palisade. The fibrovascular stroma is fine. The outline of the central cavity is irregular. It extends among lobules of neoplastic cells and contains a variable amount of necrotic debris and proteinaceous material. The neoplastic spindle cells have small to moderate amount of eosinophilic to amphophilic cytoplasm, indistinct cytoplasmic margins and oval finely granular nuclei with multiple small nucleoli. There is mild anisocytosis and anisokaryosis. In some areas, the cells are polygonal and have more abundant eosinophilic and often granular cytoplasm (some of these cells appear degenerate). The mitotic rate is approximately 0-2/HPF. The tumor compresses the surrounding tissue including the sublingual salivary gland and bundles of skeletal muscle. Mostly in the connective tissue around the tumor, there is moderate multifocal mononuclear infiltration and accumulation of some pigment-laden macrophages (hemosiderin, presumptive). In the outer region of the mass, there is multifocal fibrosis, which in some areas merges with a compression capsule. At the periphery of the mass there is multifocal prolapse of neoplastic lobules into dilated veins (so-called pseudoinvasion).
Morphologic Diagnosis:
Lab Results:
Condition:
Contributor Comment:
In laboratory mice, myoepitheliomas are not uncommon but are usually limited to specific strains. The latest reference we found describing a relatively large group of these tumors in mice is Sundberg JP et al. Vet Path 28:313, 1991 which discusses myoepitheliomas diagnosed through routine surveillance of The Jackson Laboratorys production and research colonies. This is a study of 142 tumors in mice the majority of which were less than one-year-old. The following data is from this reference.
Myoepitheliomas occur spontaneously in strains A, Balb/c and their F1 hybrids. Most tumors were of salivary gland origin and the most common location was the ventral neck (74%), as in the submitted case. Other locations included head (periorbital), perineum and ventral abdomen with origin from the salivary, Harderian, clitoral, preputial and mammary glands. The gender predisposition differed between the two strains. Balb/c females were predominantly affected whilst in A/J strain the reverse was true. In the Jackson study, the mean age at which tumors were detected was 234 days but other studies indicate that tumors arise predominantly in mice which are more than one-year-old. In the Jackson colony the frequency of malignancy, as determined by pulmonary metastases or invasion of underlying bone, was low (6%). A metastatic rate of >10% is reported in other studies of older mice, suggesting that the frequency of metastasis increases with age. Myoepitheliomas have been transplanted, but their etiology remains unknown. Virus particles were not identified in ultra-structural examination and molecular studies did not produce evidence implicating viral infection.5
The macroscopic and histologic appearance of myoepitheliomas appears to be fairly consistent and similar to the submitted case. 5 Grossly, the tumors were fluctuant, ranged in size from 0.5 - >4 cm in diameter and the single large central cavity contained opaque, odorless, pink to brown watery fluid. According to The International Classification of Rodent Tumors1, the tumors are composed of pleomorphic cells that can resemble epithelial or mesenchymal cells. Adjacent to vessels, tumor cells tend to align themselves in an epithelial fashion. Multiple necrotic areas are present and mucoid material accumulates in pseudocysts as result of degeneration. The tumors can be invasive into surrounding tissue, and large tumors can metastasize to the lung.2
Immunohistochemical analysis showed that normal acinar and ductal myoepithelial cells and neoplastic cells were positive when stained with antibodies against cytokeratin, K5 and K14. Alpha-smooth muscle-1 antibody stained normal acinar myoepithelial cells but neither normal ductular myoepithelial cells nor neoplastic cells. This supports the notion that the tumors originate from ductular myo-epithelial cells.5 Normal myoepithelial cells and neoplastic cells were negative for antisarcomeric actin, panmyosin, desmin, and S-100.5
JPC Diagnosis:
Conference Comment:
Myoepitheliomas can have a somewhat variable histomorphology but are typically composed of pleomorphic spindle cells that have features of both mesenchymal and epithelial cells.4,5 As their name suggests, myoepithelial cells are contractile with both a smooth muscle and epithelial component, and myoepitheliomas are thought to be derived from the myoepithelial or ductal reserve cells of the salivary gland.3,4,5 Normally, myoepithelial cells are located between the lumen and basal lamina of secretory structures, such as salivary glands, sweat glands, lacrimal glands, and mammary glands. The epithelial component of this neoplasm can form ducts or form nests of keratin-filled horn cysts, although that is not a striking feature in this case.3,4,5 Myoepitheliomas have been reported infrequently in mammary glands in dogs, nonhuman primates, and humans. In mice, myoepitheliomas have been associated with salivary, mammary, preputial, and Harderian glands.4
Conference participants agreed that the tumor is arising from the salivary gland based on the glandular or ductular profiles occasionally found in the neoplasm interpreted as preexisting salivary structures entrapped by neoplastic cells. As noted by the contributor, there is significant slide variation in this case. This variability is due to the use of tissue from different parts of the neoplasm.
References:
1. Mohrs U, ed: International Classification of Rodent Tumors: The Mouse. Springer: Berlin; 2001:29.
2. Moumen M, Criche A, Cagnet S, Petit V Raymond K, Faraldo M, Dugnier MA, Glukohva MA. The mammary myoepithelial cell. Int J Dev Biol. 2011; 55:763-771.
3. Munday JS, Lohr CV, Kiupel M. Tumors of the alimentary tract. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: Wiley Blackwell; 2017:853-855.
4. Percy DH, Barthold SW. Mouse. In: Pathology of Laboratory Rodents and Rabbits. 4th ed. Ames, IA: Blackwell Publishing; 2016:114-115.
5. Sundberg JP, Hanson CA, Roop DR, Brown KS, Bedigian HG. Myoepitheliomas in Inbred Laboratory Mice. Vet Pathol. 1991; 28:313-322.