Signalment:
Gross Description:
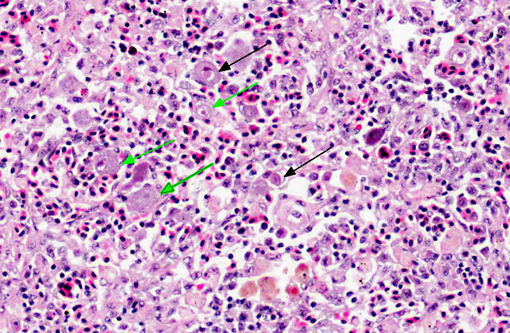
Histopathologic Description:
Morphologic Diagnosis:
Lab Results:
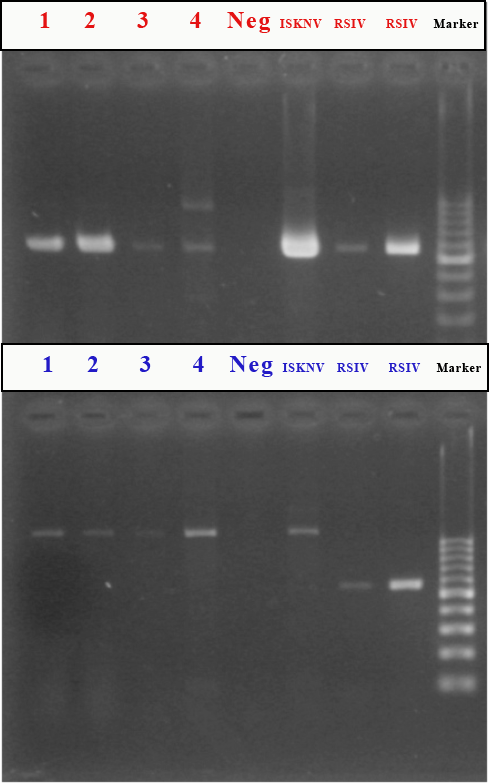
| Gel PCR on Tissue homogenate | Gel PCR on Tissue homogenate |
|
| Sample ID | RSIV1 set primers | RSIV4 set primers |
| Spleen | Pos | Neg |
| Kidney | Pos | Neg |
| Brain | Pos | Neg |
| Eye | Pos | Neg |
| RSIV1 primer |
| RSIV4 primer |
| Sample | Results |
| 1: F-09-044 Spleen homogenate | RSIV1 + RSIV4 - |
| 2: F-09-044 Kidney homogenate | RSIV1 + RSIV4 - |
| 3: F-09-044 Brain homogenate | RSIV1 + RSIV4 - |
Condition:
Contributor Comment:
Iridoviridae gets its name from Tipula iridescent virus, which grows in the haemocele of the crane fly (Tipula sp.) and makes the fly iridescent. The iridoviruses may have an envelope derived from the host plasma membrane. This membrane may not be critical for infectivity but enhances it. The iridoviruses are large isometric viruses with icosahedral symmetry, 130-133 nm in diameter, and comprise a spherical nucleoprotein core surrounded by a membrane consisting of lipid modified by protein subunits. The genome consists of a single linear molecule of double stranded DNA. Transcription and DNA synthesis are nuclear. Virion assembly is cytoplasmic.(4) Replication occurs in two stages: the first stage is in the nucleus and second stage is in the cytoplasm.(1)
Piscine iridoviruses belong to the genera Ranavirus, Lymphocystivirus and Megalocytivirus. In recent years, the genotypical variation between newly found iridovirus strains included in the genus Ranavirus has been studied in this viral family. However, the properties of and variation between iridovirus species have not been well characterized except for a few iridoviruses isolated from amphibians. Members of the Megalocytivirus genus produce characteristic basophilic inclusion bodies in the enlarged cells of host fish organs, which have been collected from mass mortalities occurring in wild and cultured fish species. Many other piscine iridoviruses have been reported in Asian countries from more than 100 different species, including freshwater and marine fish.(4)
In this case, gel PCR was performed on tissue homogenate by using two sets of primers according to the recommended World Organization for Animal Health (OIE) protocol. RSIV1 primers revealed virus in all tissues tested (i.e., spleen, kidney, brain, and eye). RSIV1 (Forward & Reverse) primers are used for the amplification of the gene sequence (570 bp) of both RSIV DNA and ISKNV DNA. RSIV4 primers were used for amplification of DNA gene sequence (open reading frame, ORF) (563 bp); however, all results were negative.(3) RSIV4 primers (Forward & Reverse) amplify RSIV, but not ISKNV DNA. These results indicated that the virus detected in these tissues is ISKNV, one of the causative agents of Red sea bream Iridovirus Disease.
The first outbreak of RSIVD was recorded in 1990 on Shikoku Island, Japan. Since that time, the disease has also been found widely in East and Southeast Asian countries, including Chinese Taipei, Peoples Republic of China, Hong Kong, Republic of Korea, Malaysia, Philippines, Singapore and Thailand. Transmission is horizontal via the water. Vertical transmission of RSIVD has not yet been investigated. Carrier states of the agents have also not yet been investigated.(3)
As in this case (which occurred in July 2009), disease outbreaks seem to occur most often during the summer months when water temperatures are 25°C or above.(3) Diseased fish are typically lethargic, show severe anemia, petechiae of the gills and hypertrophic spleens.(6) The susceptibility of juveniles is generally higher than adults.(3) The only clinical sign seen in this case was lethargy. Histological preparations characteristically show enlarged cells of the spleen, kidney, liver and gill.(4) Hematopoietic tissue is located in the stroma of the spleen and the interstitium of the kidney in teleosts. Therefore, histopathological observations are consistent with the anemia and splenomegaly observed in fish with RSIVD.(6) Not all of the fish in this group had histopathological changes and many had no lesions in any organ examined.
We found some inconsistencies in the terminology from the literature regarding the enlarged cells seen in RSIVD. Some texts and journals refer to the hypertrophied cells as having inclusion bodies, while others distinctly refrain from using that term.
JPC Diagnosis:
1. Spleen, leukocytes: Cytomegaly, diffuse, marked, with intracytoplasmic viral inclusions.
2. Mesentery: Peritonitis, subacute, mild.
Conference Comment:
In addition to RSIVD, proficiently described by the contributor, participants discussed other viruses in the familyIridoviridae that affect commercial fish production. Specifically, Lymphocystivirus spp., including lymphocystis disease virus 1 (LCDV-1) and LCDV-2, cause lymphocystis disease in many species of freshwater and marine fish.(2) These viruses infect fibroblasts in the skin, gills, and internal connective tissue and arrest cell division but not cell growth, resulting in markedly hypertrophied cells (i.e., lymphocysts) that can reach up to 100,000 times their normal size.(2) Microscopically, lymphocysts have the following distinct features: a large nucleus, a hyaline-like capsule, and bizarre and segmented cytoplasmic inclusions. Grossly they appear as raised, pearl-like lesions. Lymphocystis is generally a self-limiting disease and is rarely fatal, as fish usually slough the external lymphocysts; however, the virus does impact the fish industry due to the cosmetic effects in fish sold for food or as ornamental fish. Additionally, the lesions may provide portals of entry for secondary pathogens.(2)
Conference participants briefly discussed the nomenclature for green grouper. In addition to green grouper, Epinephelus coioides is known by several common names, including orange spotted grouper, estuary cod, and estuary rock cod.(7)
References:
2. Knowles DP. Asfarviridae and Iridoviridae. In: MacLachlan NJ, Dubovi EJ, eds. Fenners Veterinary Virology. 4th ed. London, UK: Elsevier Academic Press; 2011:172-176.
3. Office International des Epizooties Aquatic Animal Health Standards Commission: Red sea bream Iridoviral Disease. OIE Manual of Diagnostic Tests for Aquatic Animals. Chapters 2, 3, 7. World Organization for Animal Health. 2009:251-261.
4. Roberts RJ. Fish Pathology. 3rd ed. London, UK: WB Saunders; 2001:182-191.
5. Shinmoto H, Taniguchi K, Ikawa T, Kawai K, Oshima S. Phenotypic diversity of infectious Red sea bream iridovirus isolates from cultured fish in Japan. Applied and Environmental Microbiology. 2009;75:3535-3541.
6. Wang CS, Shih HH, Ku CC, Chen SN. Studies on epizootic iridovirus infection among Red sea bream, Pagrus major (Temminick & Schlegel), cultured in Taiwan. Journal of Fish Diseases. 2003;26:127-133.
7. www.arkive.org