Signalment:
Three-year-old,
male, RCS rat (
Rattus norvegicus).The rat was kept
as a non-treated animal in a long-term rat study. No clinical signs except
coarse hair were found until the scheduled sacrifice at 152 weeks old.
Gross Description:
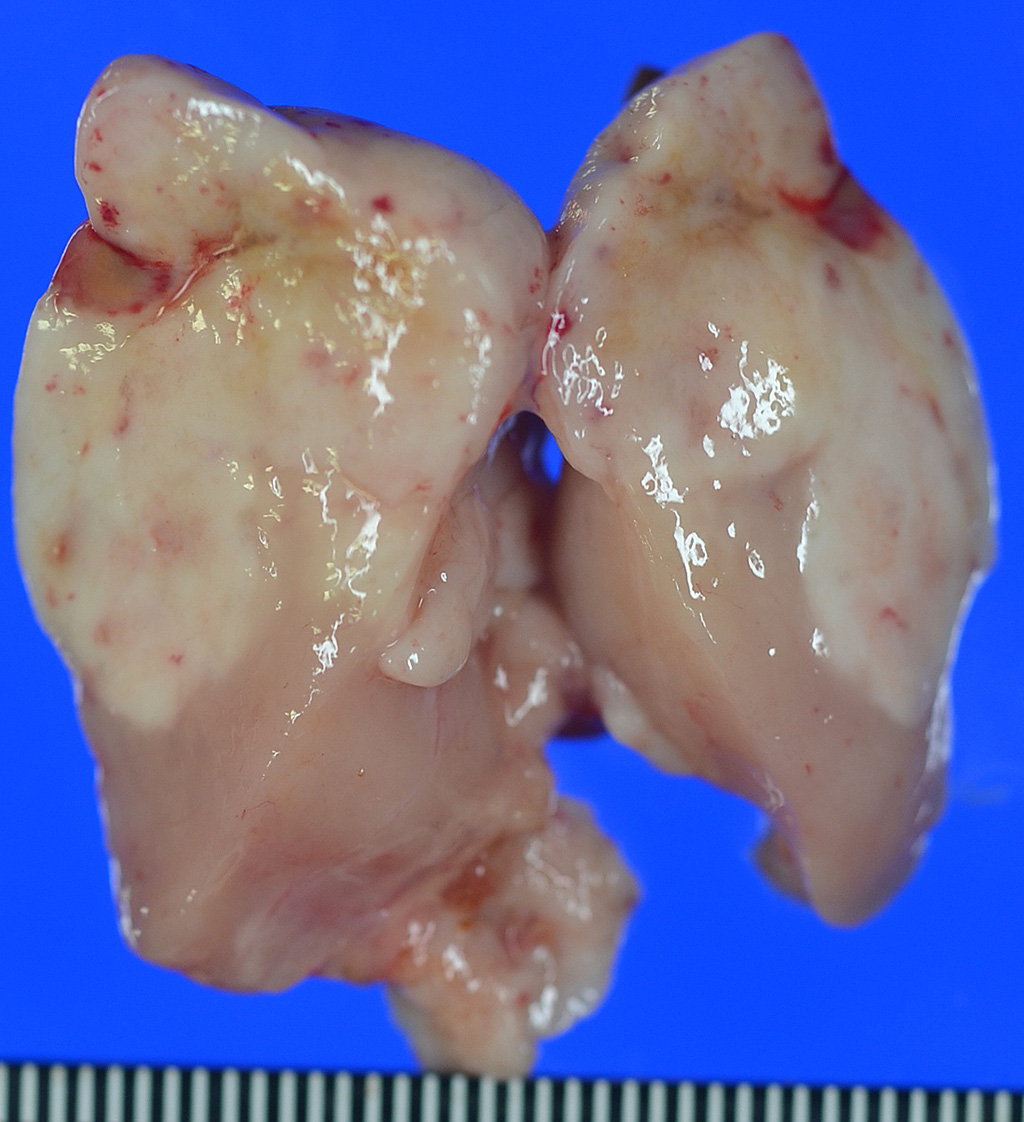
A
mass measured 40 x 30 x 15 mm, was observed in the anterior mediastinum, and
adhered to the pleura of the lung. Small masses had found around main mass, and
adhered to the lung and esophagus. The mass was soft, and the cut surface was
milky white to yellow and grey-white.
Histopathologic Description:
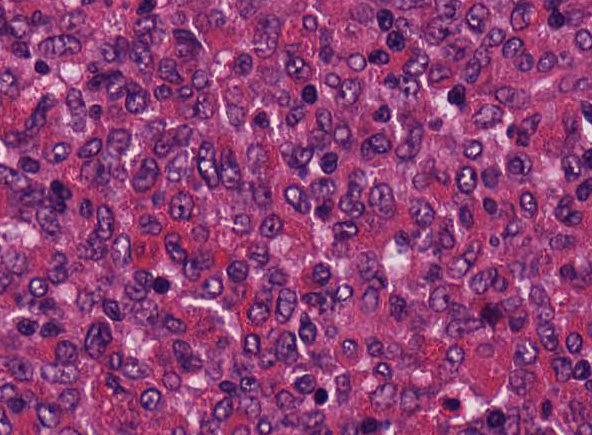
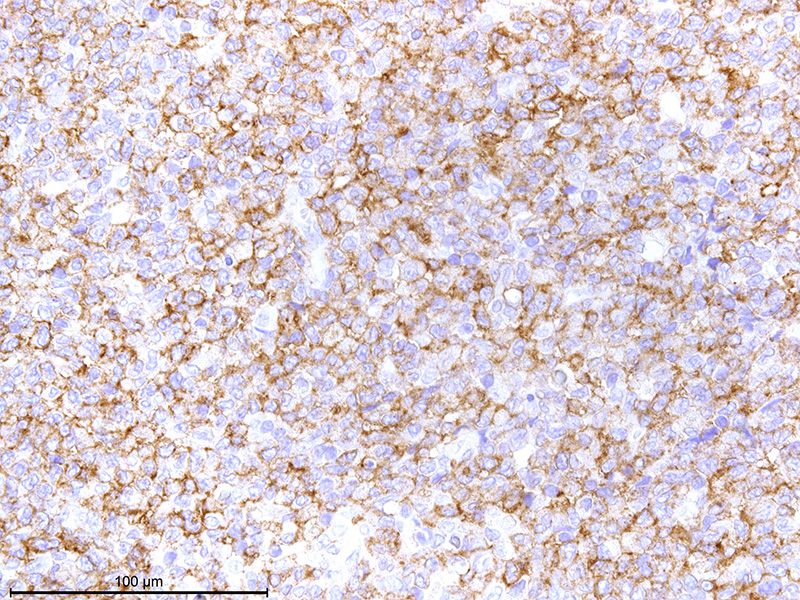
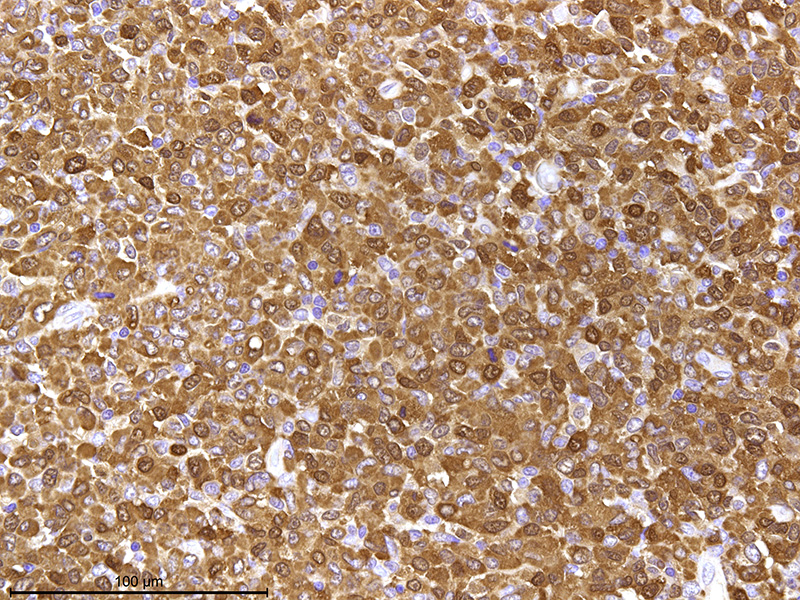
Affecting
approximately 80% of this section of thymus is an unencapsulated, poorly
circumscribed, moderately cellular neoplasm composed of round to spindle-shaped
cells arranged in sheets on a pre-existent fibrovascular stroma. Neoplastic
cells have variably distinct cell borders, an abundant amount of lightly
eosinophilic to amphophilic cytoplasm that is often obscured by fine
eosinophilic granules. The nuclei of the tumor cells are
generally centrally placed, round to elongate, with finely stippled chromatin,
and 1-2 variably distinct nucleoli. Mitotic count averages about 1 per HPF.
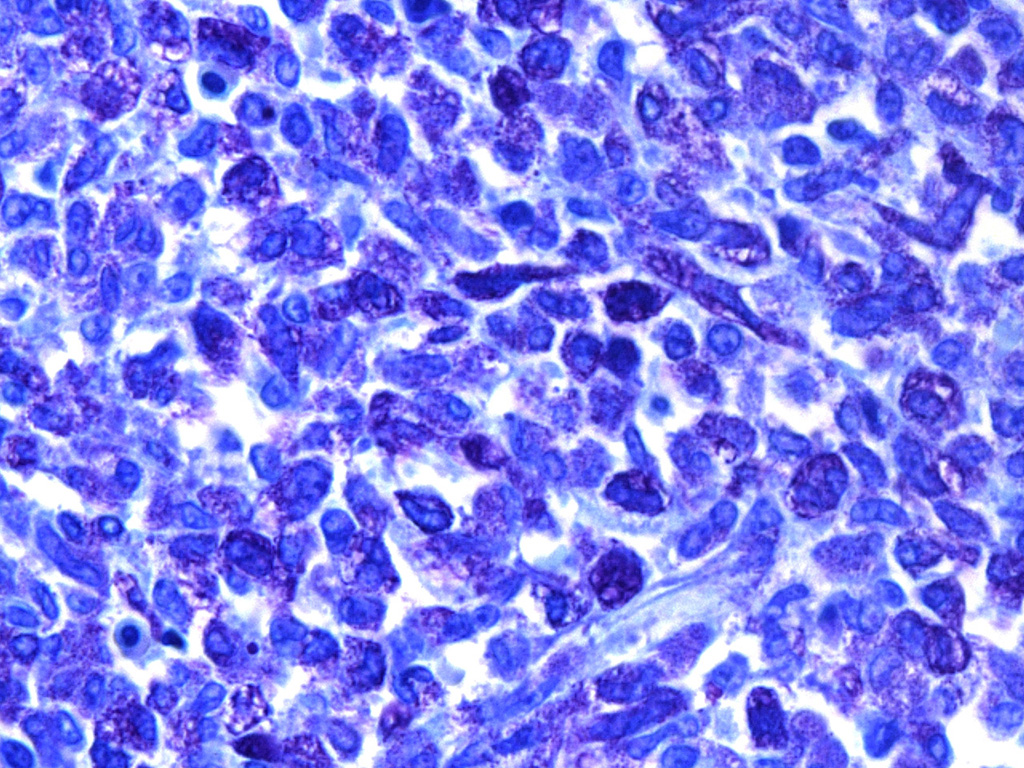
Almost all tumor cells have metachromatic granules when stained with toluidine
blue stain. Immuno-histochemically, tumor cells are positive for c-kit,
strongly positive for rat mast cell protease, and mostly negative for histamine
as mast cell tumor markers. All tumor cells are negative for cytokeratin
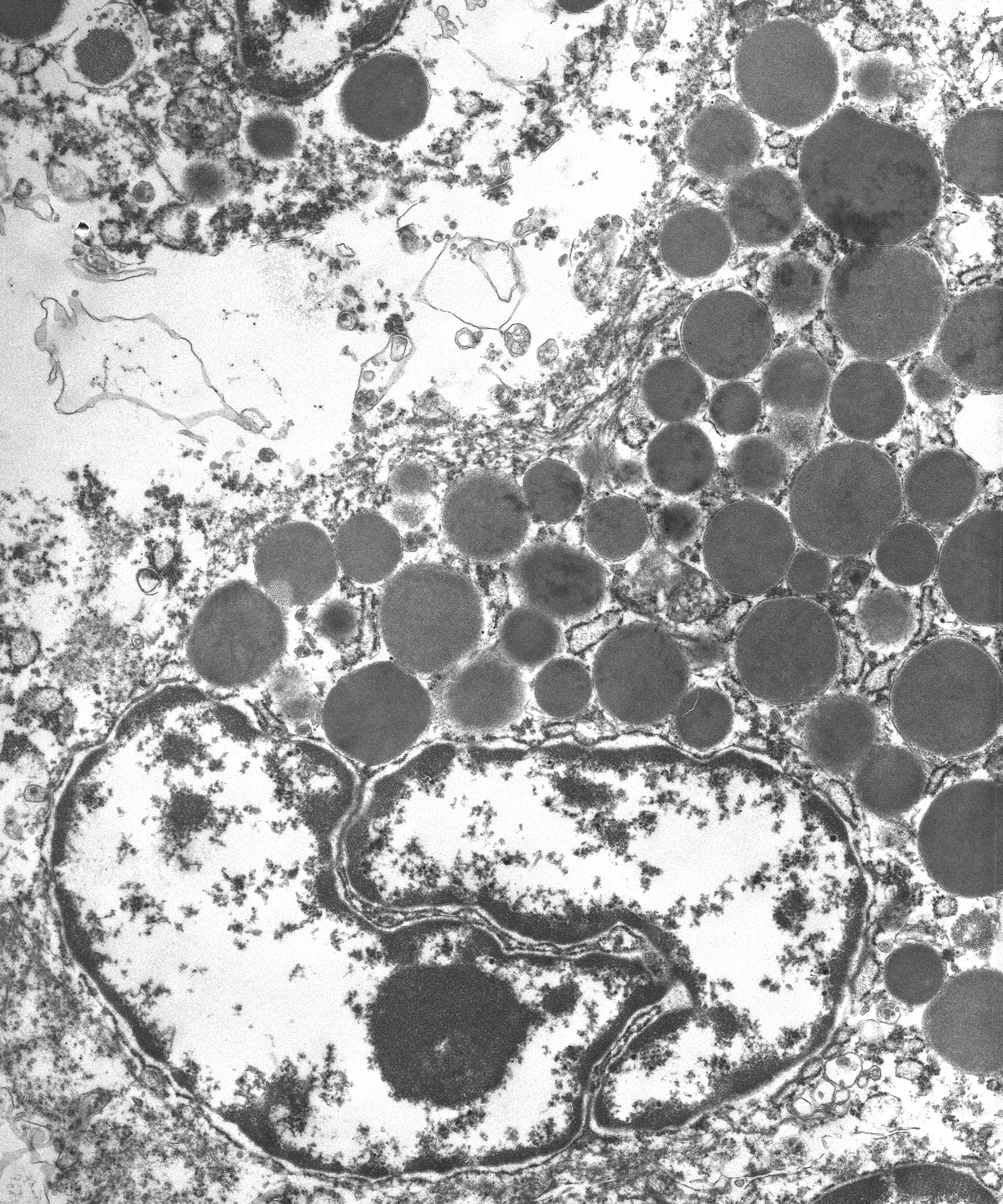
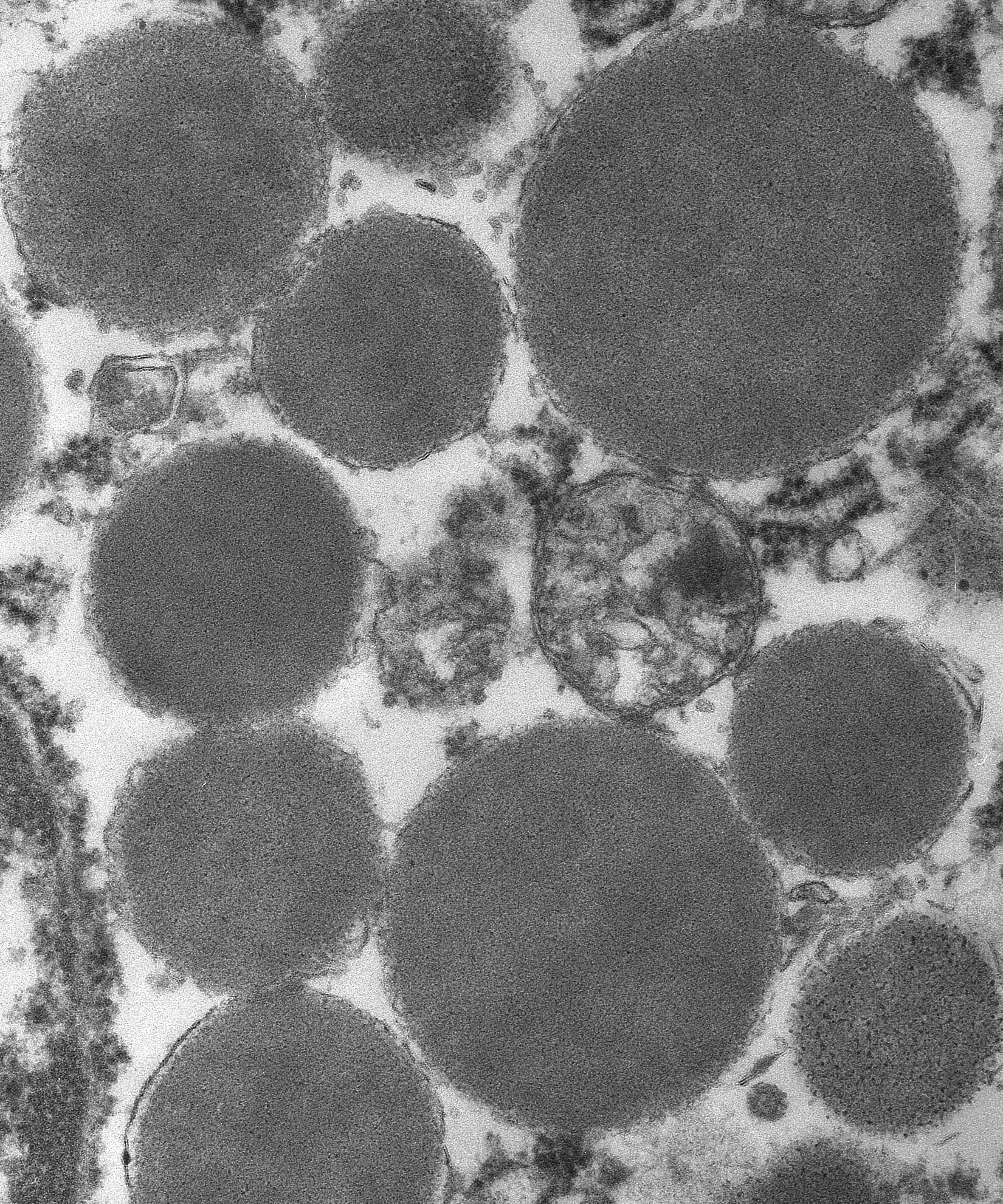
AE1/AE3, and positive for vimentin. Ultrastructurally, the various sized
granules contained homogeneous electron-dense material consistent with mast
cell granules. The tumor metastasized and disseminated to the pleura of the
lung and esophagus, and adventitia of the left ventricles and aorta.
The
basophilic area at the periphery of the mass consists of a large number of
lymphocytes and thymic epithelial cells similar to normal thymic tissue. Lympho-cytes
in high cell density areas of epithelial cells are mostly a small cell type
with coarse chromatin and fewer large lymphocytes. On the other hand,
lymphocytes in low cell density areas of epithelial cells are nearly uniform
medium-sized cell with fine stippled chromatin. Immunohistochemically, the hymic
epithelial cells in high-cell-density
areas exhibit positive cytokeratin 8 staining, which is expressed in thymic
cortical epithelial cells. Thymic
epithelial cells in low-cell-density areas are positive for cytokeratin 14
which is expressed in thymic medullary epithelial cells.
Morphologic Diagnosis:
Thymus: Malignant mast cell tumor with thymic
epithelial hyperplasia
Lab Results:
N/A
Condition:
Mast cell tumor
Contributor Comment:
This
malignant tumor of thymic origin was located in the anterior mediastinum, invaded
the adjacent thymus, and metastasized to the
thoracic cavity. The tumor is characterized by a dense round cell
proliferation, and has the features of a malignant round cell tumor. Neoplastic
cells have intracytoplasmic metachromatic granules with toluidine blue stain
and are strongly immunopositive for mast cell markers. Therefore, the tumor is diagnosed
as a malignant mast cell tumor. Mast
cell tumor is a very common neoplasm of the skin in the dog and cat
5,6,
and is composed of round cells with basophilic
granules which are metachromatic when stained with toluidine blue. With regard to
rodents, chemical and radiation-induced and spontaneous mast cell tumor have
been reported in mice.
3,8 However, to our knowledge, mast
cell tumors are extremely rare in rats. Rat mast cell tumors have only been
reported in two case reports; and there are 12 cases/entries in the National
Toxicology Program (NTP) pathology database.
2,3 Histopathologically,
the mast cell tumors described in the two case reports originated in the thymus
and in the eyelid, and they were characterized by a sheet-like proliferation of
round cells with fine cytoplasmic eosinophilic granules. However, infiltration
of eosinophils and an increase in collagen fibers are not observed, unlike in
cases of these tumors in dogs and cats.
1,2,3,4
Because
our case has similar morphologic features to previously reported cases, mast
cell tumor in the rat may be characterized by eosinophilic granules in the
cytoplasm. However, since this case has variable cell morphology and evidence
of metastasis, the mast cell tumor in this case may have more malignant
potential compared with previous reports.
This case is characterized by sheet-like
proliferation of spindle to round cells with eosinophilic granules of various sizes. Differential diagnoses for the present tumor included thymoma,
granular cell tumor, and globule leukocyte tumor. Thymoma, an epithelial tumor,
is easily distinguishable from a round cell tumor; however, the patterns of
cellular proliferation observed in the present case resemble that seen in
tumors of epithelial origin, making the rat tumor difficult to differentiate from thymoma. Negative immunohistochemical
staining for cytokeratin AE1/AE3 helps to rule out a thymoma in this rat.
Granular cell tumors and globule leukocyte tumors are characterized as round
cell tumors with eosinophilic granules, similar to those observed in the
current case. Accordingly, metachromasia, using toluidine blue, was required to
confirm the diagnosis.
2,7,9,12
The thymic region of the tumor described here is composed of
two areas with different densities, as well as different epithelial cell and
lymphocyte morphologies, suggesting that cortical and medullary thymic
components may have been maintained in the tumor. In humans, cortical and
medullary thymic epithelial cells yield different expression patterns of
cytokeratin. Medullary thymic epithelial cells express cytokeratin 5 and
cytokeratin 14, whereas cortical thymic epithelial cells express cytokeratin 8
and cytokeratin 18.
5,11 Accordingly, immunohistochemical staining
for different cytokeratins distinguishes between the cortex and the medulla of
the thymus.
In
this study, the expression patterns of cytokeratins 8 and 14 were analyzed in
thymic epithelial cells of a normal RCS rat and shown to be similar to those
observed in humans. Cytokeratins 8 and 14 were thus considered suitable markers
for distinguishing between the cortex and medulla in RCS rats. In the RCS rat
described here, the distribution of cytokeratins 8 and 14 corresponded to the
areas with high and low epithelial cell densities, respectively. It was clear
that the thymic area possessed both cortical and medullary components but that
the epithelial cell density differed from that of the normal thymus. However,
growth of solid tubules and epithelial cords, which represent a characteristic
feature of benign thymoma, is not observed in our case. The area exhibiting high
epithelial cell density represented the cortical component of the tumor with
thymic epithelial hyperplasia; accordingly, this region was diagnosed as thymic
epithelial hyperplasia. In our laboratory, we previously detected only one
benign thymoma in about 20 RCS rats of over 120 weeks of age; however, almost
all thymus showed severe involution. Therefore, this strain may not be prone to
the development of thymic epithelial hyperplasia and thymoma.
JPC Diagnosis:
Thymus: Mast
cell tumor, RCS rat,
Rattus norvegicus.
Conference Comment:
Conference participants had great difficulty with the diagnosis and tissue
identification in this case. While most attendees agreed that this case
represented a malignant round cell neoplasm, none had mast cell tumor as a
differential or thymus as the affected tissue. The neoplasm effaces the
majority of the tissue; however, normal thymic parenchyma is present at the
periphery of all examined tissue sections. As a result, the conference moderator
led a discussion of the anatomical features of the rodent thymus.
The thymus
is located in the mediastinum, cranial and ventral to the base of the heart and
aortic arch with extension into the cervical region in the rat. It consists of
two bilateral lobes joined by a connective tissue isthmus. Within the lobe, a
thin capsule surrounds each lobule and gives rise to septae; however, septation
is not apparent in this section.
10,11
The thymus is unique among the lymphoid organs
because it is supported by an epithelial framework, highlighted by the
contributors cytokeratin immuno-histochemical stains. It is divided into a
cortex and medulla separated by a vascular corticomedullary zone.
Histologically, the darkly staining cortex contains densely packed, small,
immature T-lymphocytes, which obscure the epithelial cell population.
10,11
The medulla is less densely cellular than the cortex, and
contains more mature T-cells, prominent epithelial cells, macrophages,
dendritic cells, and B lymphocytes. Hassalls
corpuscles are rare in rodents when compared with many other species, contributing
to the difficulty participants had in tissue identification. In addition, given
the age of this rat, the tissue is likely in an advanced stage of involution,
further obscuring the normal architecture.
10,11
Neoplastic cells in
this section have numerous prominent pale eosinophilic cytoplasmic granules
which are inconsistent with the deeply basophilic granules seen in
well-granulated mast cell tumors in dogs and cats, as well as in normal rat
mast cells. Conference participants considered other differentials including
undifferentiated malignant round cell neoplasm, granular cell tumor,
oncocytoma, balloon cell melanoma, and large granular lymphoma. In addition to
the histochemical and immunohistochemical stains mentioned by the contributor
that support the diagnosis of mast cell tumor, the provided transmission
electron microscopy (TEM) image nicely demonstrates numerous intracytoplasmic
homogenous electron dense granules consistent with mast cell granules.
References:
1. Amihai
D, Trachtenburg S, et al. The structure of mast cell secretory granules in the
blind mole rat (
Spalax ehrenbergi).
J Struct Biol. 2001;
136:96-100.
2. Baselmans AH,
Kuijpers MH, van Dijk JE. Brief communication: Histopathology of a
spontaneously developing mast cell sarcoma in a Wistar rat.
Toxicol Pathol.
1996; 24: 365-369.
3. Haseman JK,
Hailey JR, Morris RW. Spontaneous neoplasm incidences in Fischer 344 rats and
B6C3F1 mice in two-year carcinogenicity studies: A National Toxicology Program
update.
Toxicol Pathol. 1998; 26: 428-441.
4. Hosseini E,
Pedram B, Bahrami AM, Moghaddam MH, Javanbakht J, Ghomi FE, Moghaddam NJ,
Koohestani M, Shafiee R. Cutaneous mast cell tumor (mastocytoma): Cyto-
histopathological and haematological investigations.
Diagn Pathol. 2014;
9:9.
5. Lee EN, Park JK,
Lee JR, Oh SO, Baek SY, Kim BS, Yoon S. Characterization of the expression of
cytokeratins 5, 8, and 14 in mouse thymic epithelial cells during thymus
regeneration following acute thymic involution.
Anat Cell Biol. 2011;
44:14-24.
6. Misdorp
W. Mast cells and canine mast cell tumors: A review.
Vet Q. 2004;
26:156-169.
7. Miyajima R,
Hosoi M, Yamamoto S, Mikami S, Yamakawa S, Iwata H, Enomoto M. Eosinophilic granulated
cells comprising a tumor in a Fischer rat.
Toxicol Pathol. 1999;
27:233-236.
8. Miyakawa Y, Sato
SI, Kakimoto K, Takahashi M, Hayashi Y. Induction of cutaneous mast cell tumors
by N-methyl-N'-nitro-N-nitrosoguanidine followed by TPA in female mice of 4 out
of 5 strains tested.
Cancer Lett. 1990; 49:19-24.
9. Nagatani M,
Nakamura A, Yamaguchi Y, Aikawa T, Tamura K. Spontaneous eosinophilic
granulated round cell tumors in rats.
Vet Pathol. 2001; 38:317-324.
10. Pearse G. Normal
structure, function, and histology of the thymus.
Toxicol Pathol. 2006;
34:504-514.
11. Sun L, Li H, Luo
H, Zhao Y. Thymic epithelial cell development and its dysfunction in human
diseases.
Biomed Res Int. 2014; 206929.
12. Yamagishi Y,
Katsuta O, Tsuchitani M. Mastocytoma in a Fischer 344 rat.
J Vet Med Sci.
1992; 54:783-785.